Placenta Model
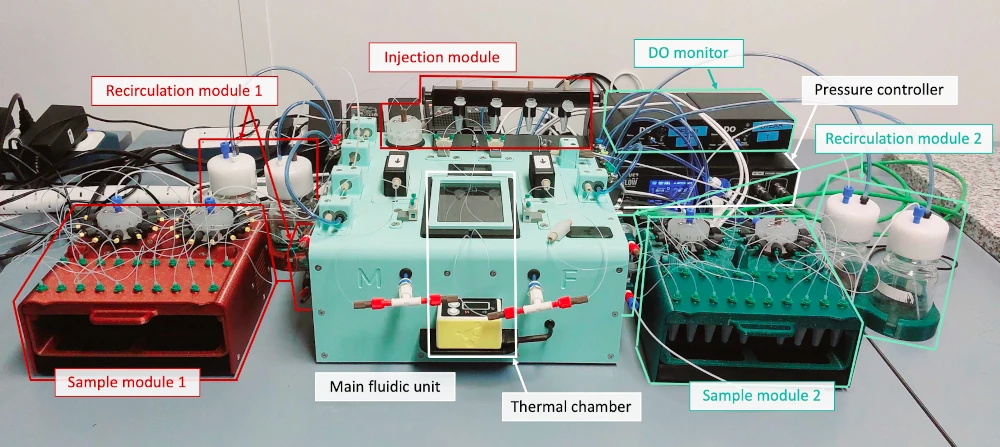
Automoated and parallelized cell culture platform for long-term cell perfusion
Completely automated platform
Less manual work and longer experiments
Independent microenvironments
Adjust the requirements for each side of the membrane
Time-resolved results
Unravel the evolution of molecular transport

Need a microfluidic SME partner for your Horizon Europe project?
Placenta model
But increased resolution usually also means increased complexity, and these systems can easily become difficult to reproduce anywhere else but at the lab where they were conceived.
Reproducibility and robustness are crucial topics to consider when choosing a technology to perform experiments with, so our team developed a platform that encompasses and automates all necessary functionalities of a barrier model experiment, in this case, focusing on the molecular transport between mother and fetus in a placenta model.
Placenta model Setup
- a recirculation system to perfuse the cells continuously, with a fail-safe mechanism in case of clogging;
- a temperature controller to keep everything at 37ºC;
- O2 sensors to monitor the microenvironment;
- Sample collector for automated and time-resolved collection;
- It can plug in any cross-membrane chip connecting both sides;
The schematic below represents the mother side of the placenta model, and the working principle of the recirculation and collection. The fetus side is exactly the same, but parameters can be adjusted to better mimic the fetus development.
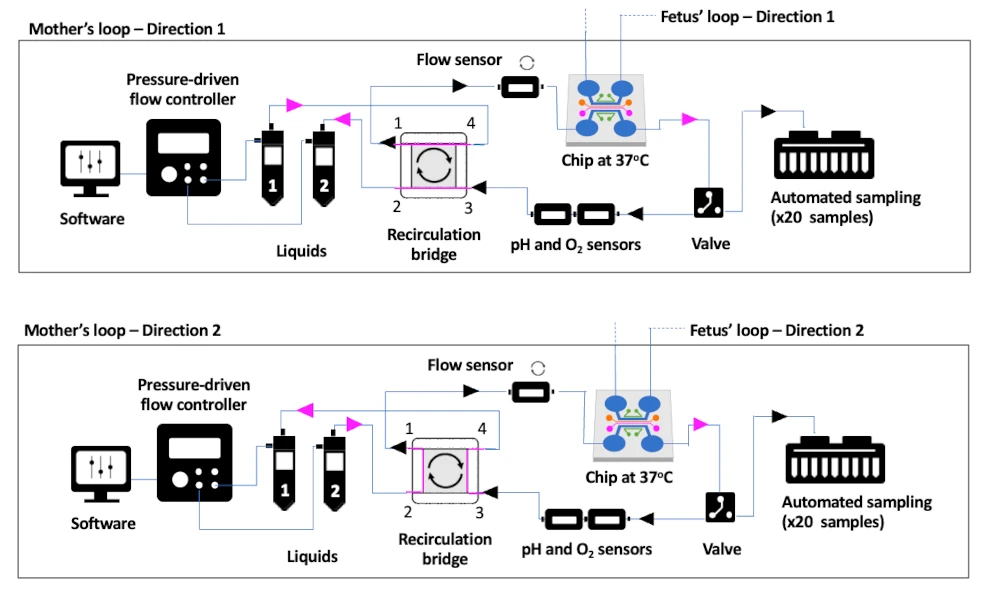
The control of the functionalities is centralised in a dedicated software.
References
Featured image: Mouse-Fetus and placenta, 2008. Wei Hsu, Shang-Yi Chiu, Lessons on Life from SENP2 Sedwick C PLoS Biology Vol. 6, No. 12, e312 doi:10.1371/journal.pbio.0060312
Compatibility and applications
Our placenta model platform can be used as a barrier model to study molecular transport in other applications, such as:
Gut-on-a-chip pack
Intestinal cells coculture under flow, mimicking the gut physiology
✓ All microfluidic pieces included, quick and easy assembly
✓ Dynamic culture conditions
✓ Advanced in viro/ex vivo
Gut-on-chip
Inflammatory bowel disease model
Automatically collect important markers of IBD in a relevant in vitro model
✓ Uncover cytokine profile changes in time
✓ Mimic pathological conditions of IBD
✓ Tailor sample volume to your analysis
Inflammatory bowel disease model
Blood-brain barrier on chip
Plug-and-play instrument pack for long term BBB on a chip study
✓ Relevant microenvironment
✓ Automatized organ-on-chip perfusion
✓ Plug-and-play microfluidic platform
Blood-brain Barrier on Chip
Liver-on-a-chip pack
Mimic the liver microenvironment in long term experiments
✓ Improve your reproducibility with physiological culturing conditions
✓ Automated and controlled supply of nutrients in a stable flow
✓ Test different conditions at the same time
Liver-on-chip
Lung-on-a-chip pack
Perform lung research in a physiologically relevant microenvironment
✓ Culture your lung cells in a physiological air-liquid interface
✓ Continuous and controlled supply of nutrients in a stable flow
✓ Stop losing your cell experiment due to clogging
Lung-on-a-chip
And many more!
References
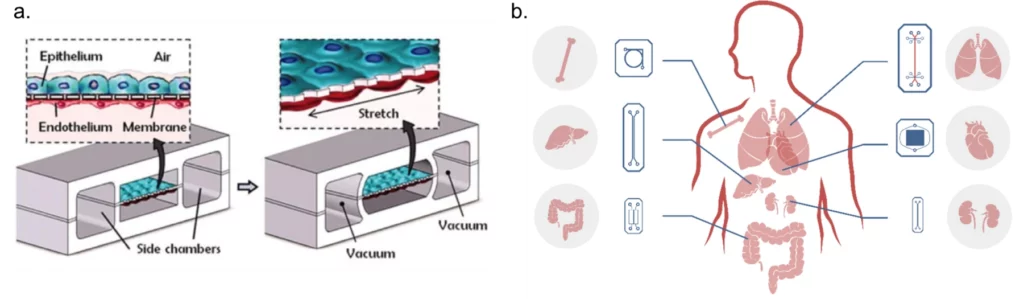
a. Adapted from: Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668.
b. Designed internally
Placenta model recirculation system
The technical specifications of the pump and valves of the recirculation system for the placenta model are:
| Characteristics | Specifications |
|---|---|
| Accuracy | +/- 2.5 mbar |
| Flow rates | 0-5ml/min depending on flow sensors |
| Air consumption | few ml/min |
| Response time | 140 ms |
| Settling time | 2750 ms |
| Overshoot | 0.12 mbar |
| Recirculation Bridge | Internal volume: 4 ml/loop |
Automated sampler
| Characteristics | Specifications |
|---|---|
| Number of samples | Up to 20 samples per side |
| Volume of collection vial | 1,5 to 2 ml Eppendorfs |
O2 sensors
| Components | Technical specifications |
|---|---|
| Wetted Material | PTFE |
| Dimensions | 10x10x10 cm (control unit) 3x1x1 cm (sensing unit) |
| Admissible Flow rates | 1-100 µL/min |
| Accessible Oxygen Levels | 0-20 %DO |
| Stability of the control | +/- 0.5 %DO/td> |
| Dynamic range of control | 0.5% DO / min |
| pH range | 6-8 pH |
| Stability of the control | +/- 0.5 pH |
Frequently asked questions
How many chips can we run at the same time?
Currently, the platform can hold only one cross-membrane chip.
What are the characteristics of the membrane of the chip?
The platform was designed to host most types of the chip, commercial or home-made, with the right adapters. So the membrane tech specifications depend on your choice of material.
Can the platform be placed inside the CO2 incubator?
No, the placenta model platform was designed to be independent of the incubator.
Do I need to use a specific chip?
No, the platform was designed to host most chips, whether commercial or home-made, with the right adapters.
Do you provide the chips with the cells already grown inside?
No, we provide the fluidic circuit and automation to run a variety of barrier model experiments with this platform. However, the biological part is out of our scope. You will need to develop and culture your cells yourself and we can help you add your biological model to the platform.




Funding and Support
The LIFESAVER and Micro4Nano projects helped develop this instrument. These projects are funded by the European Union’s H2020-LC-GD-2020-3, grant agreement no. 101036702 (LIFESAVER) and H2020-MSCA-RISE-2020, grant agreement no. 101007804 (Micro4Nano).
Products & Associated Accessories
FAQ - Placenta model
In simple terms, what is this placenta model platform?
The placenta model is an automated, parallelized cell culture platform designed for long-term cell-perfusion studies of molecular transport between the mother and fetus. It’s a barrier model system that replicates the interface between maternal and fetal compartments, allowing researchers to study how molecules cross the placental barrier in a controlled, reproducible environment.
What does two independent microenvironments mean in this case?
Mother and fetus are physically separate but linked by a cross-membrane chip. Each side is recirculated and has its own settings. Practically, it can be used to set conditions differently on either side (flow, oxygen setpoints, sampling rhythm), which is generally required if you want to model asymmetric physiology rather than imposing an average environment.
What are the key building blocks on one side of the model? What are the key building blocks on the other side of the model?
Each side includes:
A continuous perfusion recirculation system, including a fail-safe feature to reduce the clogging in the experiment.
Temperature regulation at 37 °C.
Oxygen sensors.
A time-resolved sampling automated sample collector.
A cross-membrane chip (you choose which kind of chip) interface plug.
Maintenance is not decentralized across multiple autonomous devices under control, but rather through dedicated software.
What is the maximum number of parallel chips?
The design currently includes only one cross-membrane chip. (Multi-chip is commonly requested immediately; the intent here is to make the most out of robustness and the depth of automation of a single barrier interface as opposed to dividing attention into a number of semi-automated channels.)
Am I obliged to use a certain membrane or a dedicated chip?
No. Most types of chips -commercial or homemade- are supposed to work on the platform as long as you have the appropriate adapters. As a result, it does not impose membrane properties (material, pore size, thickness, etc.), since they vary depending on the chip you choose to use for your biology and readouts.
Can it fit in a CO2 incubator?
No. The platform of the placenta model is meant to be independent of the incubator. The temperature is regulated and controlled at 37 °C within the system; the environment is measured using sensors (not assumed to be in an incubator). When your workflow is highly dependent on the location of the incubator then it is worth reconsidering the logic of the setup: the system is designed to act as more of a regulated bench-top organ-on-chip station than as a passive incubator insert.
What are the technical aspects of the recirculation system (flow, response, volumes)?
Some figures that are likely to be significant when designing the method:
Flow rates: 0 5 mL/min (depending on the flow sensors used)
Pressure accuracy: +- 2.5 mbar
Response time: 140 ms
Settling time: 2750 ms
Overshoot: 0.12 mbar
Internal volume: 4 mL/loop (recirculation bridge)
Such are the parameters that silently determine whether long-term perfusion remains stable or gradually deviates into inexplicable variability.
Oxygen (and other microenvironment control) management?
The wetted material of the O2 sensing/control modules is PTFE, and the cover is compatible with flow rates of about 1-100 uL/min at the sensing unit. Available oxygen concentrations are 0-20% DO, and the control stability is specified as ±0.5 % DO, with a dynamic control range of approximately 0.5% DO/min. The same module family implies a pH range of 6-8 and is stable in +-0.5 pH. That is, you should not take oxygen as a background condition but as a real experimental variable.
Can it be restricted to placenta research on this platform?
Not really. It is a barrier-model automation platform, with the placenta as a flagship application. The same platform can also be used for gut-on-a-chip-style work, inflammatory bowel disease models with programmable marker collection, blood-brain barrier studies, liver microenvironment work, and lung models (when the chip architecture supports it). No single organ label is the common denominator; the combination of two sides + transport + long perfusion + reproducible sampling.
What do you provide- and what you do not provide- when one purchases/uses this pack?
The platform is based on fluidic circuits, automation, sensing/monitoring, and instrument-side reliability, enabling long experiments to succeed. The biological layer (cell sourcing, differentiation, seeding, ECM selection, and validation assays) is the user’s responsibility. With that said, MIC can assist you in hooking your biological model into the platform- in practice, that usually entails co-designing adapters, chip interfaces, sampling plans, and protocols that are failure-proof, so that on day 10, you do not lose a run due to a clog on day 6.
When I am putting together a Horizon Europe proposal, it would be irrelevant to me whether MIC is a microfluidic SME or simply a supplier.
A good SME partner usually affects the plausibility of the work plan: prototyping, manufacturability, integration, risk mitigation, and the non-glamorous (but decisive) elements of such work, such as automation and repeatability. The positioning of MIC is quite practical: it designs robust microfluidic systems for research, produces/industrializes chips as needed, and provides operational prototypes.

