LEVEL SENSORS FOR MICROFLUIDICS
Continuous, unidirectional flow over samples with low volumes of fluid
Less manual work to maintain your long term recirculation
Fully autonomous and automated; stress-free assembly
No more losing your experiment due to clogging
Level sensors

Automated & secured recirculation system
Most automated recirculation systems rely on the initial fluid volume and desired flow rates to estimate the time for one reservoir to flow most of its volume through the sample and then switch to the other reservoir. In cell culture, for example, as cells grow and proliferate, they release debris that can foul and clog the lines, changing the flow rates.
Even small changes can affect the time it takes for one reservoir to empty. However, the system is usually insensitive to this change. Thus, it continues to switch between reservoirs as programmed.
If one of the reservoirs empties before was expected due to biofouling or clogging, it introduces air into the system and damages the cells, ruining the experiment.
Take full advantage of controlled recirculation
To avoid this problem, our microfluidic level sensors add an extra layer of responsiveness to your system and ensure that your reservoirs are never empty, so your experiment is always full of liquid, and you don’t risk having air passing through your chamber and disrupting your sample.

The level sensors automate your system based on the actual media volume level inside your reservoirs, so changes in flow rate due to fouling are accounted for, and the time it takes to switch between reservoirs is adjusted.
In case something goes wrong, for example, leakage due to extensive clogging of the lines, the level sensors will stop the flow and preserve your sample.
Automation and control with a single software interface
User-friendly and intuitive software to get started in a few clicks and further automate the most complex and long-lasting experiments. If you need more flexibility, SDK libraries can control the system with your code and involve third-party instruments.
Level sensor applications for dynamic cell culture
A great asset of a cell perfusion system is providing a more physiologically relevant cell microenvironment by sustaining a constant unidirectional flow through a microfluidic chip laden with cells. In this light, limited volumes of liquid flow back and forth between two reservoirs, as seen above.
The media flow subjects cells to relevant shear stress while providing a fresh supply of nutrients at a constant level of O2 and CO2 while flushing away waste, all with a high level of control. The level sensors add an extra layer of control by introducing a fail-safe mechanism that considers the changes that can happen within such a dynamic system, such as fouling the tubing with the debris of cellular death, leakage due to clogging, and other matters blockage-related as the entry of air.
As illustrated below, the level sensors can be added to our automated cell culture platform or to a cell perfusion system quickly built with our available instruments.
Examples of setups using level sensors
1) Automated cell perfusion platform (Principle)
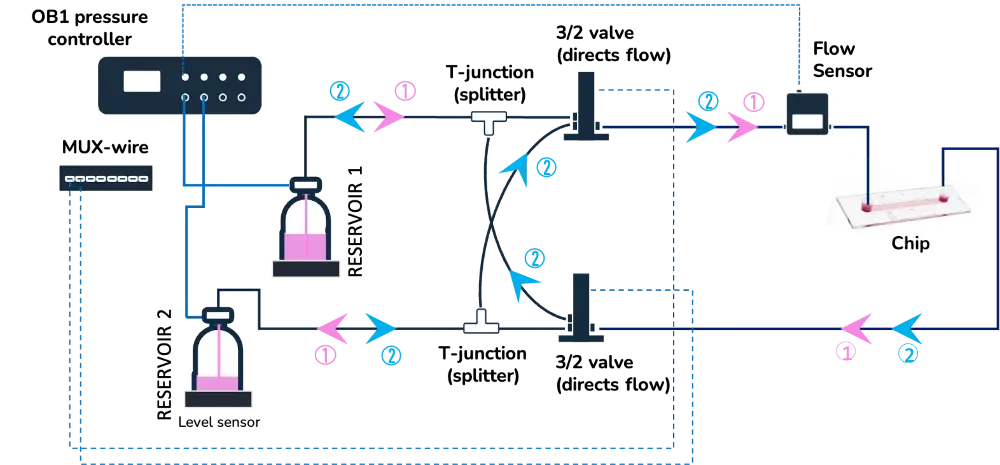
2) Cell perfusion pack with MUX recirculation

3) Cell perfusion pack with active valves
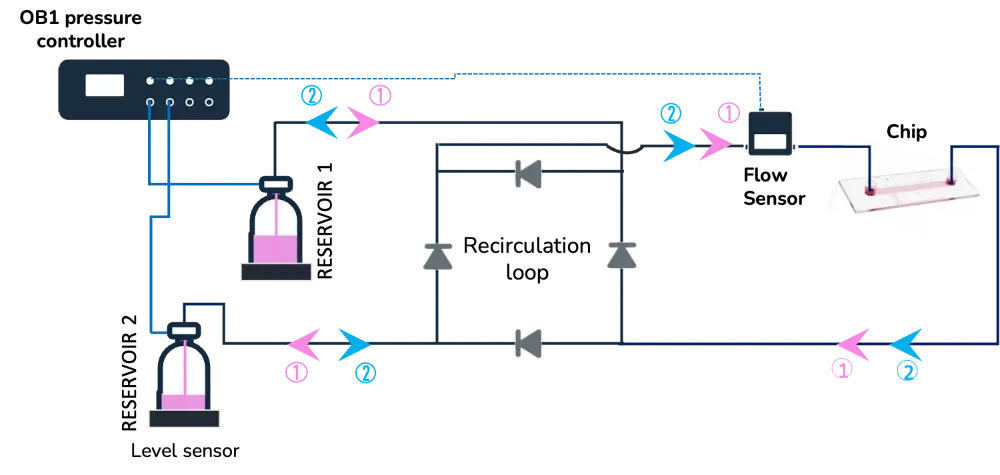
These examples include the following instruments:
| 1. Automated cell perfusion platform | 2. MUX recirculation | 3. Active valves |
OB1 flow controller (Elveflow) Recirculation loop Flow sensor (Elveflow) | OB1 pressure controller (Elveflow) Flow sensor (Elveflow) MUX recirculation (Elveflow) | OB1 pressure controller (Elveflow) MUX wire (Elveflow) Flow sensor (Elveflow) 3/2 valves |
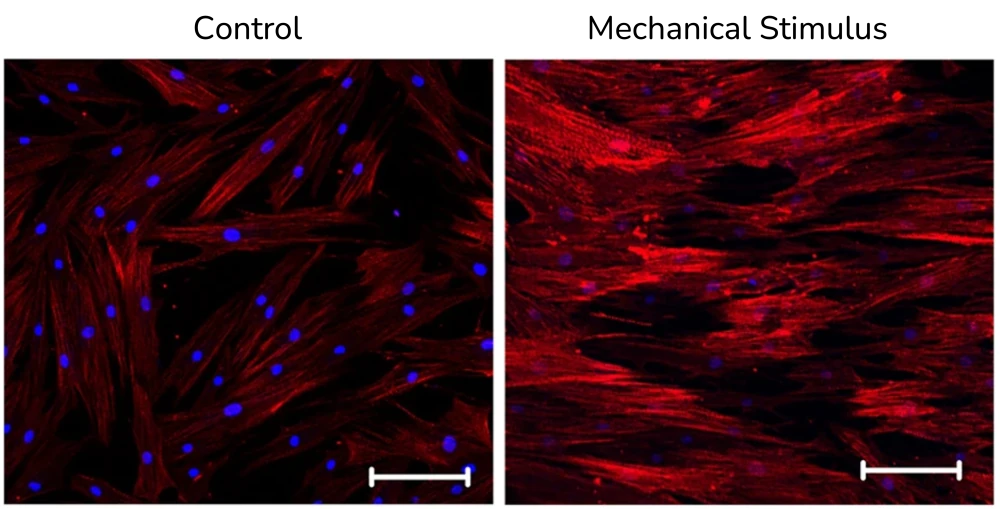
Some biological applications of our microfluidic level sensors include:
We have built expertise in microfluidic flow control for over ten years to provide you with state-of-the-art microfluidics systems. From biology to engineering applications, the Microfluidics Innovation Center is the perfect partner for you to get started with microfluidics.
Level sensor pack specifications

The following table summarizes the main specifications of the level sensors for microfluidics. This is a product subjected to optimization.
Component |
Technical specifications |
| Dimensions (cm) | Sensor: 2x1x0.27 cm; S (15 ml holder: 10x7x3 cm); M (50 ml holder: 10x7x4 cm) |
| Material | Plastic |
| Reservoir compatibility | 15 and 50 ml Falcon tubes |
| Control | OB1 + Elveflow ESI |
The level sensors are compatible with standard incubator conditions (37oC, 5% CO2, and 100% humidity). The liquid being sensed should be able to conduct current for proper functionality.
Customize your pack
Our instruments can benefit from your feedback, so you can take advantage of the extra flexibility to adapt them depending on your specific needs. Our microfluidic specialists will advise you on the best instruments and accessories depending on your needs and will accompany you during the setup of the microfluidic platform.
All the instruments are controlled by the same ESI software, allowing workflow automation and easy integration in your program with free available libraries.
– Check our other Packs for various applications –
Is the instrument compatible with other reservoirs than Falcon tubes?
The current version is compatible with Eppendorf or Falcon tubes, but other options are being developed, such as for well-plates. If you need a different type of reservoir, contact us!
What extra equipment is needed?
Besides the fluid flow equipment, such as the pressure-driven flow controller, flow sensor, and chip, you will need a computer to run the software to program the automated sample collection.
Does it only work with experiments under flow?
The automated sampling unit needs to be connected to a flow generator so the automation of the process is possible. Thus, it is best adapted to microfluidics and perfusion experiments. The experiments can have continuous or intermittent (stop/go) flow.
Funding and Support
The LIFESAVER project helped develop this instrument. This project is funded by the European Union’s H2020-LC-GD-2020-3, grant agreement No. 101036702 (LIFESAVER).

