LIVE CELL IMAGING
Keep your cells at 37°C ± 0.5°C outside the CO2 incubator
Test different conditions in the same system
Designed for plates, dishes and perfused chips
Live cell imaging under perfusion
Our stage top incubator allows you to efficiently perform long-term live cell imaging experiments on top of the microscope stage. Designed to fit k-frame microscope stages, the internal chamber fits up to three parallel chips. The ITO glass ensures homogeneous temperature across the microfluidic devices, and the frame was made so you don’t need to worry about heating your reservoirs. Your media will arrive at the right temperature on top of your cells regardless of whether you leave the reservoir at room temperature.
How does it work?
As a proof-of-concept of the suitability of the stage top incubator for live cell imaging, we cultured mouse microglial cells for 48 hours under continuous perfusion on top of the microscope stage. As shown below, the media flow control was done with the OB1 pressure-driven flow controller and MFS flow sensor.
Microglial cells, like other cell types from the brain parenchyma, are susceptible to shear stress as they are not usually exposed to flow in their physiological environment. Thus, to prevent the negative effects of shear stress, we employed the µ-Slide III 3D Perfusion and a microfluidic resistance to ensure a low and stable flow.
Live cell imaging pack setup
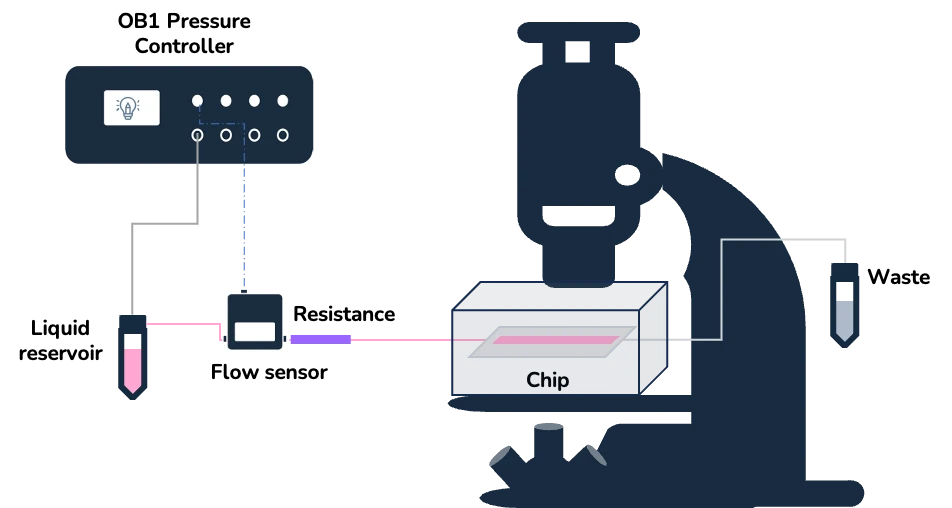
The live cell imaging pack includes:
- Stage top incubator
- OB1 pressure-driven flow controller (Elveflow)
- MFS flow sensor (Elveflow)
- u-Slide III 3D Perfusion
Details of the experimental design can be found on the Specifications tab.
Experimental details
According to the protocol published by Sepulveda et al., Glia, 2016, primary microglial cells were isolated from mice pups’ brains.
Primary mouse microglial cells were prepared and kindly provided by Patrick P. Michel and Rocio Gimenez from the Brain and Spine Institute in the frame of the LOCAI project.
After one week of isolation, cells were passaged onto ibidi chips (µ-Slide III 3D Perfusion, cat. 80376) at 150 000 cells/mL density. Cell medium was supplemented with FBS and PS. HEPES in powder was added to a final concentration of 20 mM. Once the HEPES dissolved, the pH was corrected and brought to 7.2 using Na 1 N, and then the media was filtered using a 0,22 filter. Cells were left to attach overnight in an incubator at 37°C and 5% CO2 before live cell imaging.
Technical specifications
Hardware
- OB1 MK4 flow controller (Elveflow)
- 1 Flow sensor MFS3 (Elveflow)
- Tubings (1/32″ ID), fittings and reservoirs
- IBIDI µ-Slide III 3D perfusion
- 8 cm of 100 µm inner diameter microfluidic resistance
- Microfluidic male and female Luer to ¼” -28 connectors
- Laminar flow hood
- Standard Incubator for cell attachment, stage top incubator for experiment
Chemicals
- Dulbecco’s modified Eagle’s medium (DMEM)
- Penicillin/Streptomycin, 10,000 U/ml, 10mg/ml Streptomycin, PAN biotech
- FBS, 0.2 µm sterile filtered, PAN Biotech
- HEPES, to buffer pH in the absence of CO2 control.
Software
- ESI software
- Thermal chamber software
Chip design
| Interface type | Female Luer |
| Well diameter | 5.5 mm |
| Well height | 1.7 mm |
| Volume per well | 30 μl |
| Material | Ibidi Polymer Coverslip |
| Surface treatment | ibiTreat |
| Growth area per well | 25 mm2 |
Live cell imaging results
Live cell imaging allows for studying the dynamic aspect of cellular processes. In vitro models, however, need to be under strict environmental control. Parameters such as pH, temperature, and oxygen levels are critical for robust and reliable results. Whether the experimental paradigm requires shear stress, continuous perfusion, or static conditions, the need for accurate temperature regulation is the same.
The videos below show 8h time-lapses of primary microglial cells taken immediately after lipopolysaccharide (LPS) stimulation. These videos were recorded using microglial cells seeded onto microfluidic chips and either left under static, non-perfused conditions (video 1) or connected to a pumping system for continuous perfusion along the experiment (1 µL/min, video 2).
Video 1. Microglial cells were seeded in a µ-Slide III 3D Perfusion chip well and maintained in the stage-top incubator on the microscope frame in static conditions for 8 hours after LPS stimulation. Cells were challenged with 10 ng/mL LPS at t = 0 h. Micrographs in phase contrast were acquired every 30 minutes for 8 hours. Image acquisition was made using an AxioVert inverted microscope with a 20x magnification objective (Zeiss).
Video 2. Microglial cells were seeded in a µ-Slide III 3D Perfusion chip well and maintained in the stage-top incubator with continuous perfusion at a 1 µL/min flow rate. Cells were challenged with 10 ng/mL LPS at t = 0 h. Micrographs in phase contrast were acquired every 20 minutes for 8 hours. Image acquisition was made using an AxioVert inverted microscope with a 20x magnification objective (Zeiss).
In both cases, the incubator allowed for the recording of microglial cells. Micrographs taken every 20 or 30 minutes show these cells’ highly dynamic and motile nature. Significantly, microglial cells, like other cell types from the brain parenchyma, are not exposed to mechanical stresses under physiological conditions. They require nutrient delivery and removal of waste metabolites for survival as in physiological conditions.
The stage top incubator presented here allows for flexibility in designing experiments, including when deciding to apply specific mechanical stress linked to flow. Several off-the-shelf chips with different geometries will allow us to extend the design possibilities to address physiological or pathophysiological conditions using the tissue or cell type of choice.
Customize your pack
Do you use well-plates instead of microfluidic chips for live cell imaging? Your microscope doesn’t have a k-frame stage? You don’t work at all with cell biology but need to keep your samples warm on top of the microscope stage? No worries! Just email us and we will see what we can do!
Our Packs can be modified depending on your specific needs. Our microfluidic specialists will advise you to provide the best instruments and accessories, depending on your needs. They will accompany you during the setup of the microfluidic platform.
– Check our other Packs for various applications –
What are the dimensiosn of a K-frame stage top?
For all the specifications of the stage top incubator and its compatibilities, please check the dedicated page here.
Can I order a pack?
Our Packs, such as the Live Cell Imaging pack, are available under specific conditions. As these packs are still in the developmental stage, we have some eligibility criteria to ensure their optimal success rate.
At the Microfluidics Innovation Center, we tailor our approach to suit each researcher’s unique needs. Therefore, before purchasing a Pack, we encourage you to contact our technical team for a detailed discussion of your research objectives and requirements.
To learn more about our Packs or to schedule a consultation with our experts, please contact us at innovation@microfluidic.fr. We look forward to collaborating with you and supporting your research endeavors.
Can I buy individual instruments?
You can order our instruments on the product section of our website.
Is the stage top incubator gas-tight?
No, the Stage Top Incubator allows gas exchange with the atmosphere.
Funding and Support
The ALTERNATIVE and LIFESAVER project developments helped develop this instrument pack. These projects are funded by European Union’s H2020-LC-GD-2020-3, grant agreements No. 101037090 (ALTERNATIVE) and 101036702 (LIFESAVER).