Nanocarriers for nonlinear microscopy: Micro4Nano
Author
Christa Ivanova, PhD
Publication Date
November 30, 2021
Keywords
nonlinear microscopy
Multifunctional nanocarriers
bioimaging
Raman microscopy
3D visualization
two-photon microscopy
drug delivery
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
New tools for biology and medicine.
Multifunctional nanocarriers for nonlinear microscopy: introduction
Multi-photon microscopy using nanocarriers enables the 3D visualization of large sections of biological tissues to understand the relationship between their structure and function, leading to applications in biomedicine and drug delivery.
Micro4Nano aims to establish and optimize new tools, materials, and techniques for the 3D functional imaging of thick tissues to extract reliable information about the tissue structure, biochemical composition, and function.
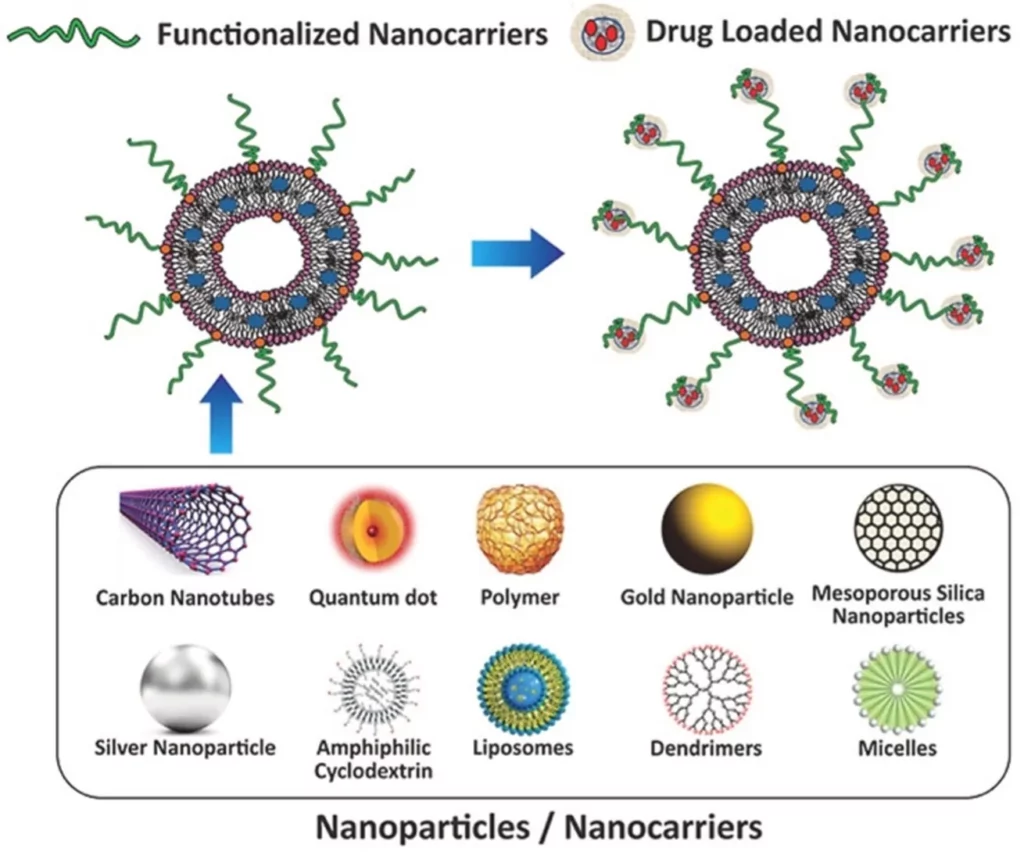
Multifunctional nanocarriers for nonlinear microscopy: project description
Micro4Nano will design, optimize, and produce a new generation of highly efficient, photostable, biocompatible, and fluorescent nanocarriers for bioimaging. This unique tool will allow for multicolor detection and consequently enable the reconstruction of complex and functional images in the study of drug delivery.
In addition, this highly ambitious project aims to develop methods for 3D visualization up to 1 mm in depth, combining hyperspectral imaging of auto-fluorescent species with structural information from second-harmonic generation signals and multifunctional fluorescent labels, as well as chemical information from Raman microscopy.
Micro4Nano will also create a new generation of multifunctional nanocarriers for two-photon microscopy, and drug delivery will be designed for real-time monitoring of living cells and tissues.
In this consortium of 11 partners, the Microfluidics Innovation Center (MIC) brings its expertise on cell culture in microfluidics devices to design tissue-on-chip platforms. We will primarily participate in designing and testing microfluidic setups for automated tissue, defining flow control parameters to reproduce the native cell environment optimally.
The highly reproducible tissue culture environment will be exploited to study drug delivery at the single-cell level using Raman and two-photon microscopy.
1. A. Shah, S. Aftab, J. Nisar, M.N. Ashiq, F.J. Iftikhar “Nanocarriers for targeted drug delivery”. Drug Deliv. Sci. Technol., 62 (2021), Article 102426.
Related content & results from this project
As a first result of the Micro4Nano project, the MIC developed the PMMA device station.
Then, we have developed:
- The thermoplastic molding pack
- Polystyrene molding setup
- Polycarbonate molding setup
- COC molding setup.
In addition, we have published a review comparing different bidirectional and unidirectional recirculation systems and an application note about perfusion cell culture using our recirculation system.
Funding
This project has received funding from the European Union under H2020-MSCA-RISE-2020, grant agreement no. 101007804 (Micro4Nano).
Start date: 1 October 2021
End date: 30 September 2026
Overall budget: € 795 800. 00



Check our Projects
FAQ – Nanocarriers for nonlinear microscopy: MICRO4NANO
What is Micro4Nano?
Micro4Nano is a European Union-funded project that aims to develop a new generation of fluorescent, biocompatible nanoparticles and imaging technology to visualize them deep within tissues.
What is the scientific problem?
Thick tissues are difficult to read: the scattering, autofluorescence, and limited penetration all degrade image quality. Micro4Nano addresses this by designing photostable labels that are bright and complementary to each other, so that function (e.g., drug release), composition (hyperspectral and Raman), and architecture (SHG) can be mapped concurrently to depths of the order of millimetres.
What about the workflow development?
The workflow development is in 3D visualization (to approximately 1 mm into tissue) with preservation of functional contrast, which cannot be maintained in most prior wide-field or confocal pipelines without clearing or sectioning.
So what are the nanocarriers?
They are thoroughly trackable delivery vehicles that are fluorescent (to allow tracking) and multifunctional to be highly photostable and biocompatible when multiphoton-excited, and with multicolor readout capabilities (they can be multiplexed). Theoretically, this is based on the directed drug-delivery readings (e.g., Shah et al., Drug Delivery and Translational Science and Technology, 2021; article 102426), however, with photophysics adapted to nonlinear microscopy.
Which imaging modalities are used together - why is this combination so?
- Two-photon excitation provides deep and localized excitation with reduced out-of-focus background.
- Hyperspectral imaging is used to unmix fluorophores and endogenous autofluorescence.
- SHG to retrieve label-free structural signals (e.g., collagen).
- Chemical fingerprint Raman microscopy.
They are collectively capable of co-registering anatomy, chemistry, and nanocarrier dynamics on a single stack at the 3D scale.
What is MIC involved in, and who is involved?
This consortium has 11 partners. The Microfluidics Innovation Center (MIC) is the leader in microfluidic cell-culture microfluidic tissue-on-strip design, automated perfusion systems, and microfluidic flow control to recapitulate a native-like microenvironment. The two-photon and Raman readouts of single-cell drug delivery are then investigated in that controlled milieu.
Any tangible results so far?
Yes. Its initial products are a PMMA device station and a family of thermoplastic molding toolkits (polystyrene, polycarbonate, COC) for speedy, reproducible chip manufacturing. We also put out an article reviewing uni- vs. bi-directional recirculation systems and an application note describing perfusion cell culture on our recirculation platform- handy when you want constant shear and nutrient delivery in long-term experiments.
What are the implications of this on practical studies of drug delivery?
With success, you can track nanocarriers in intact perfused tissues in real time using multicolor tracking with spectral capabilities to distinguish signals from autofluorescence. It implies fewer artifacts during slicing/clearing, more accurate pharmacokinetics, and a plausible path to quantitative uptake/release measurements at the single-cell level.
Can an external lab/company be involved?
Yes, this project was designed to work collaboratively. MIC is also used to develop tissue-on-chip prototypes with consistency, automate fluid handling, and align imaging needs with microfabrication limitations. In the case of European consortia, the inclusion of a specialized SME such as MIC is usually more successful in terms of proposal success and translational milestones, since we integrate hands-on microfluidic engineering with proposal writing assistance and exploitation planning.
What value would MIC add if we submit a bid to Horizon Europe for bioimaging or delivery?
(1) Engineering – we design and fabricate strong chips and perfusion stations (that imaging teams can actually use in everyday life); (2) Science– we install single-cell assays, calibrations, and flow regimes such that the data can be defensible; (3) Funding– our group has been involved in numerous EU consortia. Among our experiences, consortia including a prototype-first SME, such as MIC, achieve a proposal success rate about 2 times higher than the official baseline on similar calls; the disparity is more evident in milestones, reasonable TRL ramps, and reviewers able to glimpse the device.
What would be a sensible initial step to determine fit?
Begin with 30-60 minutes of scoping your biological targets and imaging considerations (excitation wavelengths, NA, working distance), then map flow conditions, chip materials, and sensor readouts. When there is alignment, we prepare a mini-workplan that includes deliverables, TRLs, and a budget line aligned with the corresponding call.