Magnetic membranes for mechanical stimulation of cells: MaMi
Author
Emma Thomée
Publication Date
May 10, 2018
Status
Keywords
biomimetic locally-driven flows
magnetic membranes
cell mechanostimulation
biomimetic tissue models
tissue engineering
dynamic substrates
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
The MaMi project combines expertise in the field of magnetism with knowledge of bioinspired local flow control to create novel concepts and technological solutions that could revolutionize the field of microfluidics.
Microfluidics and magnetism in biomimetic locally-driven flows: introduction
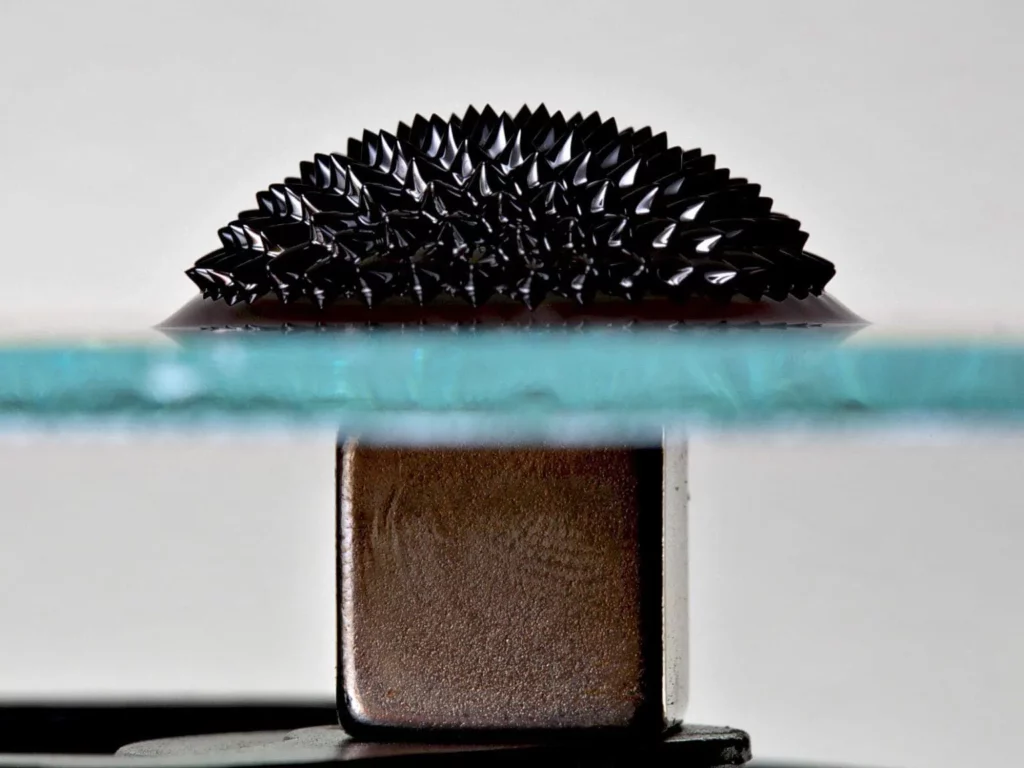
The MaMi project bridges microfluidics and magnetism research by taking advantage of magnetic forces inspired by biomimetic systems to control local flows and cargo transport.
The critical research question guiding the project is “How can magnetism and biomimetic locally-driven flows overcome the current limitations of microfluidics?”
The 9 MaMi partners will take different approaches to address this question.
Magnetic membranes for remote mechanical stimulation of cells in microfluidic devices: project description
In this consortium, we developed microfluidic devices with an industry potential for organ-on-a-chip applications.
The ultimate goal will be to address the hurdles to transitioning microfluidic techniques from prototype organ-on-a-chip devices to marketable devices.
PDMS is the gold standard fabrication material within the engineering research community. We will explore fast-fabrication thermopolymer materials (PMMA, PC, PS, COC) that are better suited for biological applications and commercially relevant with scale-up potential to design this new generation of organ-on-a-chip.
However, as thermopolymers are non-stretchable, the current methods of recreating desirable mechanical stimulation to produce more bio-relevant cellular phenotypes will not be possible.
We will, therefore, exploit magnetic forces to achieve remote mechanical stimulation within the thermopolymer organ-on-a-chip.
Related content
Have a look at these three reviews written by Emma Thomée, PhD:
Magnetic fluids and microfluidics: A short review
A short overview of lung-on-a-chip systems
Actuation concepts for in-vivo mechanical strain
Funding
This project has received funding from the European Union’s Horizon 2020 MSCA-IF under grant agreement No 766007 (MAMI).


Researcher

Emma Thomée
Research Associate
- PhD candidate at Elvesys/Strasbourg University in the frame of MSCA-ITN
- Analytical chemist (QPharma AB, Sweden)
- Master of Science in Biomedical engineering (Lund University, Sweden)
Areas of expertise:
Biomedical engineering, Microfluidics, Organ-on-chip, Magnetism.
Check our Projects
FAQ – Magnetic membranes for mechanical stimulation of cells: MaMi
What are the key restrictions or failure points to observe?
- Duty cycling (reduce thermal drift caused by heating coil).
- When you over drive the field (the field must remain within the measured loop) hysteresis in magnetization.
- Particle sedimentation as the curing (solve via viscosifiers or cure-on- rotation).
- High particle loadings (maximize filler volume fraction) Optical non-uniformity.
- Edge effects when strain fields are not evenly distributed, design your ROI far away along a boundary.
How does MAMI integrate with microfluidics or organ-on-chip?
The ceiling of a perfused microchannel may serve as the membrane. That can be used to add mechanical strain, controlled biochemical cues (perfusion, gradients) and online sampling. In barrier models, cyclic strain can frequently enhance tight-junction maturation; in fibrotic models, pathway-specific responses can be identified in tonic versus pulsatile loading. MIC regularly pairs such membranes with pressure-based flow controllers and media switching which is software-scripted.
Does it have the ability to accommodate microscopes and incubators?
Yes. The membrane chips are fabricated on optically transparent substrates and are pre-fabricated to fit into any standard holders (glass-bottom dishes, multi-well plates or custom frames). The coils may be put either around the objective or underneath the stage; with live-cell imaging low-profile coils should be used and active cooling employed, or stimulation bursts can be run short in order to eliminate focal drift. In imaging, lightweight coil frame provides improved access to long incubations, whereas in imaging large objects, magnetic setups are preferred.
What is the way to make the magnetic composite biocompatible?
The magnetic filler is incorporated into a medical grade silicone matrix; contact surfaces between the device and cells are passivated (plasma, ECM coatings) as are normal PDMS devices. Teams are usually tested with viability of 24-72 h, morphology and proliferation test (sham assay and stimulated assay). Leachable can cause assays to fail so add a bake-out and a lengthy media pre-soak, and to long term cultures, parylene or thin hydrogel interlayers should be considered.
Is the platform able to scale to multi-well screening?
Yes, within reason. In the case of medium throughput we pattern arrays of small membranes using a standard multi-well lid and coil them using a common coil and local flux concentrators. It is not yet practicable to achieve 384-well density with true independent waveforms but 6-24 conditions, parallel, and with different amplitudes or times is achievable, at least, and keeps imaging quality and temperature constant.