Artificial Intelligence and microfluidics: LOCAI
Author
Christa Ivanova, PhD
Publication Date
September 14, 2021
Status
Keywords
Artificial Intelligence
Lab-on-a-chip
in vitro cell culture
automated cell culture system
image analysis
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
The project is part of the 1st German-French joint call for proposals on Artificial Intelligence launched in 2020 to contribute to the cooperation between research and digital change of Artificial Intelligence in Germany and France.
Artificial intelligence and microfluidics: introduction

Project LOCAI (Cell growth in lab-on-a-chip devices controlled by Artificial Intelligence) aims to develop a self-regulated microfluidic cell culture system that controls the growth parameters in real-time using artificial intelligence based on image analysis.
Manual laboratory experiments and protocols (cell culture included) can be a time-consuming and error-prone process, with variable outcomes and data reproducibility difficulties.
Mammalian cell culture is an indispensable tool for biomedical research and drug development, as it is often used to determine the optimal drug dose.
In addition, different combinations of drugs can be tested with variable drug concentrations, but finding the optimal settings requires exhaustive search and complex settings – even in automated systems using microfluidics, this can be very difficult to achieve.
Using artificial intelligence and microfluidics could solve this problem through targeted exploration and the ability to learn the optimal drug mixtures, growth parameters, and schedules through interaction with the cell culture.
Towards full automation of in vitro cell culture: project description
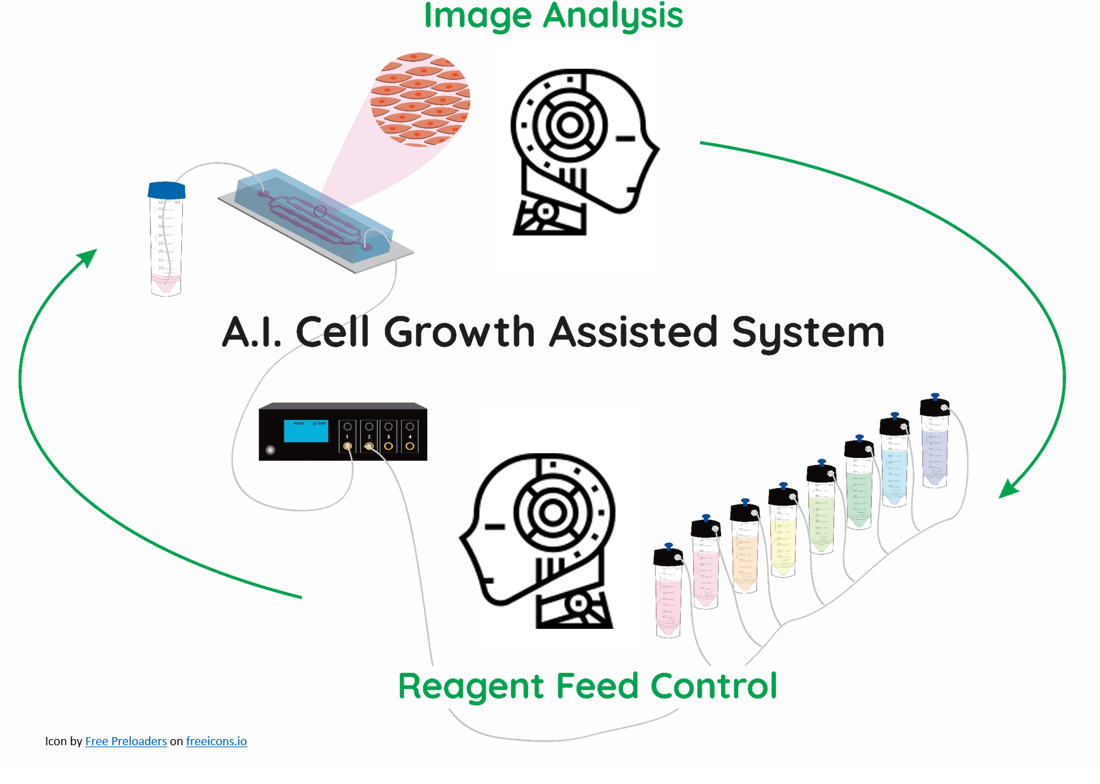
In vitro, cell culture models are regularly seen as the future of clinical testing, and consequently, their main applications are in the healthcare and pharmaceutical sectors.
Having a robust and reproducible microfluidic cell culture system will allow the reduction of animal experiments, eliminating ethical issues.
Currently, cell culture is predominantly done manually, and even in semi-automated systems using microfluidics, the user has to observe the result and adjust the growth parameters as required. Through targeted machine learning processes, artificial intelligence could detect errors and correct them automatically as efficiently as a human.
This project reunites the expertise from project partners in France and Germany, with the MIC and the Paris Brain Institute located in France, and the Biothera Institute and Freiburg University in Germany.
Each project partner will contribute to the project with its specific know-how, developing a fully automated cell culture system that combines artificial intelligence and microfluidics in a trustworthy manner.
Related content & results from this project
The LOCAI project helped develop:
- The automated sampling instrument
- The precision sampling for cell perfusion and cell culture
- The live cell imaging pack
- The stage-top-incubator
- The inflammatory bowel disease pack
In addition, we have published a review about organ-on-a-chip models and an application note about automated sample collection.
Funding





Check our Projects
FAQ – Biomaterials engineering for organ repair: THOR
What is LOCAI, in two lines?
LOCAI, or Lab-On-Chip AI, was a project completed by France and Germany that developed a self-regulating microfluidic cell-culture system. It leverages real-time image analysis and machine learning to automatically adjust growth parameters, eliminating the need for human-level monitoring.
What is the point of using AI with microfluidics to culture cells?
Because its search space is enormous, the fact that just getting a two-drug test with a couple of concentrations already yields dozens of conditions, with the addition of timing and perfusion rates, makes it easily intractable by hand. An AI-based controller can search, learn, and converge on optimal dosing schedules and environmental conditions, as the chip can maintain constant flows and gradients.
To what extent does the system in question become automated?
The project aimed to achieve full automation of in-vitro culture: imaging – inference (ML) – actuation (flow, dosing, temperature, gas) without the human in the loop for routine adjustments. The goal is human-level, 24/7 monitoring without being tired out, and microfluidic platforms can be coupled with strong control to achieve this.
What were the partners, and what did they bring?
The project brought together the complementary strengths of a microfluidics industrial partner (MIC), the Paris Brain Institute, the Biothera Institute, and the University of Freiburg. The combination of them encompassed chip engineering, cell-biology models, clinical relevance, and reliable AI/control. The combination of both is precisely what you need to make the transition from an ideal to an actually working closed-loop device.
Does this help reduce animal experimentation?
Yes. Powerful microfluidic in vitro systems, maintained with closed-loop regulation, can substitute for some animal experimentation. The project specifically aimed to reproduce chip-based models to reduce animal testing and enhance ethical and scientific standards.
Which publications or know-how outputs shall apply in the event of your wish to build upon LOCAI?
In addition to the hardware, the team created a review of the organ-on-chip model and an application note on automated sample collection – helpful resources if you are configuring similar pipelines (imaging-driven control, sample collection, and analysis).
What would this fit into my Horizon Europe proposal?
Two angles work well. Typically, the first step in any LOCAI-style closed-loop culture is to identify the disease model or screening work package that will serve as the backbone of automation. Second, de-risk timelines by using validated modules (imaging, precision sampling, stage-top incubation). In practice, involvement of an established SME in microfluidic engineering, such as MIC, enhances evaluation scores for implementation and feasibility. In our recent consortia, our involvement has increased application success by about two times the official baseline rate, because the prototype route is plausible and expeditious.
Which types of applications are the most interesting at the moment?
Directly: drug combination optimization, time-staggered responses (dose), toxicity window, and adaptive therapies, in which the system learns a control policy directly from culture images. Neurology and gut inflammation are natural products with the packs we have, but oncology and immunology follow quickly, as quickly as you desire dynamic microenvironments.
What can MIC bring on board if my lab is interested in the prototype of a similar system?
Complete microfluidic engineering, end-to-end chip design and microfabrication, continuous flow control, real-time imaging integration, and glue code of ML-in-the-loop. We also assist with writing Horizon Europe proposals, organizing the work plan, and tech valorization. Practically speaking, we include modules that are already validated in LOCAI, which means you are a step closer to a prototype that already works and that your risk (and that of reviewers) is lower.
