Recent research breakthroughs in lung-on-chip technology
Author
Marjorie Metzger
Publication Date
September 16, 2018
Keywords
Lung-on-a-chip System
disease modeling
Organ-on-a-chip
pulmonary thrombosis

Need advice for your lung-on-a-chip system?
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Introduction
In particular, organs-on-a-chip represents an innovative method that could favor health research discoveries, as Zhang et al. specified [2]. Different micromodels have already been elaborated for muscles, bones, brain, and more specifically, blood-brain barrier, gut, liver, and multi-organs systems, so it only follows that a lung-on-chip would be developed.
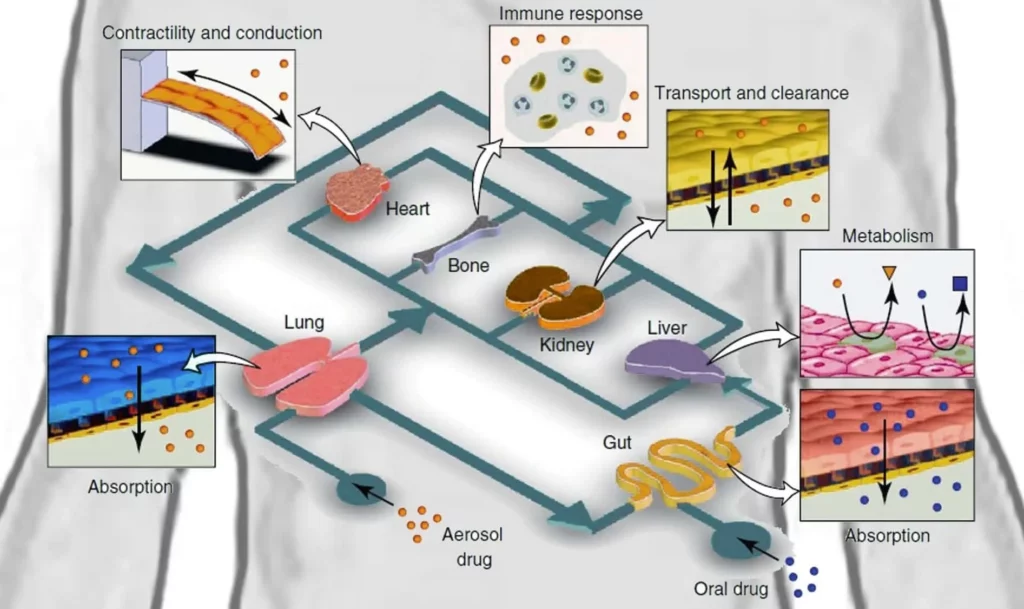
Building a human-on-a-chip (Fig. 1) that will model the interactions between different organs could be exciting. Still, developing simulations of tissue-tissue interfaces and, more generally, of local organ behavior is also essential. Fundamental exchanges happen by way of the respiration process, especially on the capillary-alveolar interface.
Reproducing lung properties on a micro-device will help understand its functions better and allow the simulation of pulmonary diseases.
Mouse models to partially reproduce pulmonary injuries exist. Still, microfluidic devices like organs-on-a-chip can exclusively model an organ’s mechanical and physiological responses or, more specifically, a tissue-tissue interface. Moreover, access to a microchip model is more accessible than that of a living mouse.
Lung-on-chip could allow more precise control of the cellular micro-environment closer to the in vivo biochemical context than the other in vitro simulations.
Challenges in the reproduction of pulmonary thrombosis in a lung-on-chip
Huh et al. [3] used the organ-on-a-chip principle to represent the alveolar-capillary interface. Indeed, the alveolar-capillary gas exchanges are essential to the lung function. Reproducing this lung unit can be helpful for nanotoxicology studies and disease simulations.
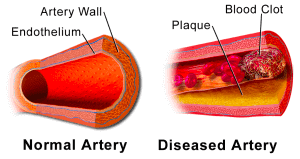
According to Jain et al. [4], pulmonary thrombosis is a significant cause of mortality in lung diseases like ALI (acute lung injury). A blood clot (Fig. 2) forms locally in a pulmonary vessel and can cause respiratory failure due to the lack of blood flow in a part of the lung.
Research is ongoing to find proper models of thrombus formation in lung vessels to develop new therapeutic drugs targeting the inflammatory mechanisms underlying thrombi formation.
However, the many animal models built to represent the formation of microvascular thrombosis in lungs are limited because they cannot be precisely similar to human lungs. Besides, experimenting on animals is under debate regarding ethics.
Research should, therefore, evolve toward efficient in vitro models. Jain et al. hypothesize that organ-on-a-chip can better represent the thrombosis mechanisms than the existing artificial models. Among other advantages, it allows easier reconfiguration and access thanks to its separate compartments.
Technical characteristics of the microdevice
Alveolar representation
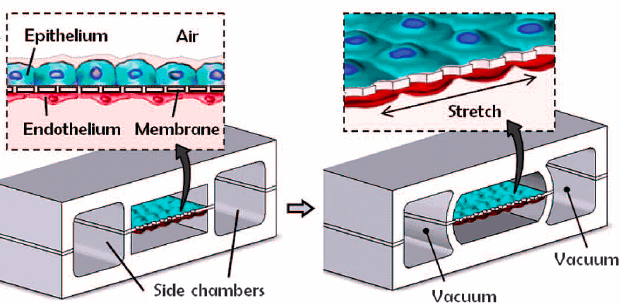
The Wyss Institute develops several pulmonary microchips [3-5]. Huh et al. [3] grew both alveolar epithelial and endothelial cells on each side of a PDMS (polydimethylsiloxane) membrane covered with an extracellular matrix (ECM) (Fig. 3).
In vivo, the ECM provides structural support and segregates tissues, the model should thus integrate them for inter-cellular communication and to avoid direct contact between cells of interest and the silicone wall.
To reproduce the alveoli, the epithelium cells were in contact with air, whereas the endothelial cells were in contact with a fluid mirroring blood properties. Two lateral chambers were also added to simulate the pressure changes in the alveoli.
Applying a vacuum to these chambers causes a deformation of the separating membrane and, therefore, models the alveolus wall’s expansion-contraction due to physiological breathing movements.
Pulmonary thrombosis model
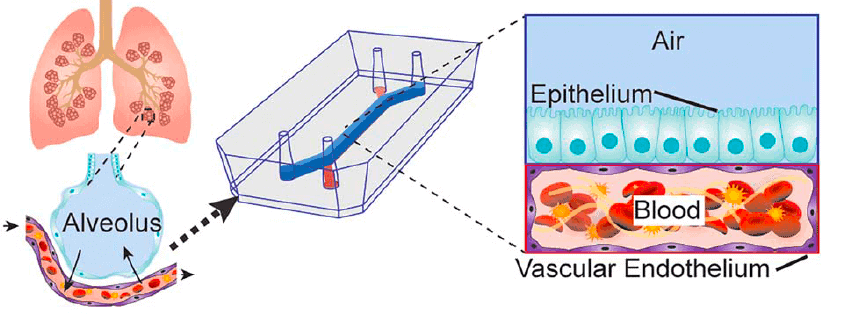
Based on previous works on lung-on-chip microdevices [3, 5], the Wyss laboratory could elaborate an innovative alveolus-on-a-chip microdevice reproducing a pulmonary thrombosis. They modified the system described before by using primary human lung cells instead of culture cells. They increased the chamber’s dimensions to have a more exposed area.
Human vascular endothelial cells from the umbilical cord (HUVEC) were added all over the bottom chamber walls to replace the fluid-mimicking plasma with real human blood (Fig. 4); in vivo, blood is not directly in contact with the extracellular matrix.
Real blood or “ersatz”?
In the microdevice developed by Jain et al., whole blood in its native state was used. Substitutes are often used instead of real blood because of technical or practical difficulties due to blood’s natural tendency to clot. Human cell blood media can mimic the principal characteristics of blood flow.
For example, Li et al. [11] used a cell culture medium with fetal bovine serum to research microvessels’ functionalities. However, the medium can only imitate blood properties. It can be an exciting approximation for some simulations, but thrombosis is a blood disease! It seems better to consider all the rheologic properties of the blood precisely.
Recalcified citrated or fresh native whole blood?
In the case studied, whole blood was first citrated to avoid excess coagulation and then recalcified. Citrated whole blood can be perfused longer than fresh whole blood without clotting. Rajwal et al. [12] found comparable parameters for both types of native-state whole blood and approved using citrated blood in clinical evaluation of coagulation states.
Lung-on-chip findings
Pulmonary thrombus formation process
Jain et al. [4] from the Wyss Institute first noticed that coating the four walls with ECM in the alveolus-on-a-chip, or lung-on-chip model, avoids platelet adhesion to the walls and thrombi formation. This microdevice can thus adequately represent a healthy pulmonary vessel behavior. The TNF-α (Tumor necrosis factor) was added to the upper chamber to simulate an intravascular thrombosis.
An inflammation-involved cytokine recruits leukocytes and activates platelets, which triggers thrombi formation [6]. It was measured by Jain et al. that the permeability of lung vessels increases proportionally to the TNF-α concentration. They also measured a rise of ICAM-1 concentration in the endothelium chamber, followed by thrombi formation on the endothelial membrane. Therefore, an in vitro model of endothelial thrombosis formation was obtained by releasing TNF-α in the upper compartment.
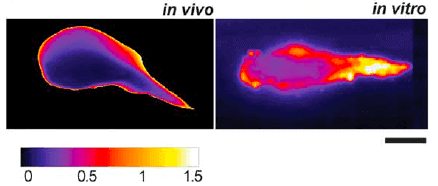
Prof. Ingber’s laboratory team [4] observed a similar shape and binding dynamic for the thrombosis model-on-a-chip compared to the in vivo laser-induced thrombi formation in a mouse model. The new model established on a microchip also demonstrates another point generally observed in vivo but never seen previously in vitro: the intra-thrombus variations due to a central kernel surrounded by a more unstable region (Fig. 5).
Jain et al. [4] tried to add LPS (lipopolysaccharides) to the lung-on-chip to demonstrate its potential for better research. LPS are endotoxins present on the outer membrane of Gram-negative bacteria. These large molecules often induce pulmonary vascular injury because they promote leukocyte recruitment [7, 8].
No thrombosis was observed at the tissue level, represented by only the vascular endothelium. On the contrary, intravascular platelet aggregates are kept at the organ level simulated by a chip with epithelial and endothelial cells.
The gap areas between endothelial cells increase after the LPS treatment. Jain et al. [4] also found an increase in three specific cytokines following the LPS addition: IL-6 (interleukin), IL-8, and monocyte chemotactic protein. They noticed similar physiological reactions in vivo: it is possible to increase the induction of lung vascular thrombosis by injecting LPS when the vessels are laser-injured.
Globally, the Wyss Institute demonstrated, using an alveolar microchip, that LPS-induced thrombosis is correlated with an inflammation process mediated by cytokines and induced by interactions between alveolar epithelial cells and vascular endothelial cells.
Using the alveolus-on-a-chip to evaluate a potential antithrombotic treatment
Once they assessed the lung alveolus chip as a proper model for thrombosis formation, they used it to test possible drug treatments. They chose to study the effects of PM-2 (Parmodulin-2) that have been shown in both in vivo and in vitro models to improve vascular fluidity and thus decrease the risk of thrombosis formation. Aisiku et al. [9] distinguished calmodulin, a family of PAR1 (Protease-activated receptor 1) antagonists, and compared them with other antagonists. They noticed that calmodulin reduces vascular inflammation without causing endothelial injury.
Based on previous studies, Jain et al. decided to analyze the action of PM-2 in their alveolus chip compared to a mouse model. Effects of PM-2 in both models match. Therefore, they demonstrate that their lung alveolus-on-a-chip model could be used for drug testing.
Further possibilities using lung-on-chip
To conclude, it has been demonstrated that a lung-on-chip with an alveolus could adequately represent the mechanical and physiological reaction of an in vivo alveolus. It allows an interesting real-time visualization of the tissue-tissue interface. Moreover, the Wyss Institute [4] integrated human blood flow into the microdevice to produce a model closer to the living processes.
They noticed good correlations between their in vitro model and the fundamental clotting dynamics in pulmonary vessels due to inflammations. Some aspects of the model can still be improved because it does not consider the breathing movement. It was already done for the alveolus-capillary interface model by Huh et al. [3].
The Wyss Institute also used HUVEC cells from the umbilical cord, whereas lung endothelial cells have some specificities, even if these endothelial cells are highly similar.
However, they could use the alveolus-on-a-chip to assess the therapeutic potentiality of a molecule. It creates opportunities for future drug assays at a lower cost and significantly higher speed. The door is also open for the modeling of other pulmonary diseases by way of microchip techniques.
Benam et al. [10] still used this method to develop a small airway-on-a-chip to mimic the physiological reactions during acute asthmatic crises. Other pulmonary complications could also be analyzed using this simple and efficient technique. Our lung-on-chip model pack illustrates how to perform such experiments in an automated and simple way.
Endothelial cells can be cultured on a chip using the endothelial cell culture Pack.
More on lung-on-chip systems:
- Lung-on-chip: origins and development
- Comparison between organ-on-a-chip, standard in vitro and in vivo tests
- A short overview of lung-on-chip systems – the technology and the potential use in human medicine
- Air-liquid interface and optimized cell culture substrate for a microfluidic lung-on-chip application
Authors:
Marjorie Metzger, reviewed by Julie Cavallasca and Guilhem Velvé Casquillas
Contact:
Partnership[at]microfluidic.fr

References
- J. A. DiMasi, H. G. Grabowski, and R. W. Hansen, “Innovation in the pharmaceutical industry: New estimates of R&D costs,” J. Health Econ., vol. 47, pp. 20–33, May 2016.
- B. Zhang and M. Radisic, “Organ-on-a-chip devices advance to market,” Lab Chip, vol. 17, no. 14, pp. 2395–2420, 2017.
- D. Huh, B. D. Matthews, A. Mammoto, M. Montoya-Zavala, H. Y. Hsin, and D. E. Ingber, “Reconstituting Organ-Level Lung Functions on a Chip,” Science, vol. 328, no. 5986, pp. 1662–1668, Jun. 2010.
- A. Jain et al., “Primary Human Lung Alveolus-on-a-chip Model of Intravascular Thrombosis for Assessment of Therapeutics,” Clin. Pharmacol. Ther., Jul. 2017.
- D. Huh et al., “A Human Disease Model of Drug Toxicity–Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice,” Sci. Transl. Med., vol. 4, no. 159, p. 159ra147-159ra147, Nov. 2012.
- P. Pignatelli et al., “Tumor necrosis factor-α as trigger of platelet activation in patients with heart failure,” Blood, vol. 106, no. 6, pp. 1992–1994, Sep. 2005.
- M. Uchiba and K. Okajima, “Antithrombin III (AT III) Prevents LPS-Induced Pulmonary Vascular Injury: Novel Biological Activity of AT III,” Semin. Thromb. Hemost., vol. 23, no. 06, pp. 583–590, Dec. 1997.
- K. Murakami et al., “Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats,” Blood, vol. 87, no. 2, pp. 642–647, 1996.
- O. Aisiku et al., “Parmodulins inhibit thrombus formation without inducing endothelial injury caused by vorapaxar,” Blood, vol. 125, no. 12, pp. 1976–1985, Mar. 2015.
- K. H. Benam et al., “Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro,” 2015.
- X. Li, S. Xu, P. He, and Y. Liu, “In Vitro Recapitulation of Functional Microvessels for the Study of Endothelial Shear Response, Nitric Oxide and [Ca2+]i,” PLOS ONE, vol. 10, no. 5, p. e0126797, May 2015.
- S. Rajwal, M. Richards, and M. O’Meara, “The use of recalcified citrated whole blood – a pragmatic approach for thromboelastography in children,” Pediatr. Anesth., vol. 14, no. 8, pp. 656–660, Aug. 2004.
- Written by Marjorie Metzger, corrected by Julie Cavallasca, under the supervision of Dr. Guilhem Velve Casquillas
Check the other Reviews
FAQ - Recent research breakthroughs in lung-on-chip technology
What is generally meant by a lung-on-chip (or alveolus-on-chip) system?
Practically, it is a microfluidic cell that aims to recreate the main functional characteristics of the alveolar-capillary interface: on one side, an air-exposed epithelial cell; on the other, a perfused endothelial cell; and in between, a thin, permeable barrier. It is not that one should literally miniaturize a whole lung. It is to isolate the functional unit in which gas exchange, inflammation, barrier leakage, and microvascular events actually occur, quantify it, control it, and repeat it.
What is so common about lung-on-chip models being mentioned in drug discovery so much?
The reason is that traditional pipelines straddle both sides: cost and translation. A recent analysis (DiMasi, Grabowski, Hansen, Journal of Health Economics, 2016) found an 8.5 percent per annum increase in the total capitalized cost of R&D (inflation-adjusted), but with a clinical success rate fixed at 10 percent over the last 40 years. Lung-on-chip does not solve that in itself, but can provide more human-relevant readouts compared to many conventional in vitro assays, particularly for barrier toxicity, inflammatory crosstalk, and vascular responses, which are not easily measured in a traditional well plate.
How is the canonical architecture of a lung-on-chip microdevice?
The most frequently mentioned blueprint involves two microchannels separated by a flexible, porous membrane (usually PDMS-based) and covered with extracellular matrix (ECM). Endothelial cells line the vascular side facing the flow, whereas alveolar epithelial cells grow on the air-facing side. Others have lateral vacuum chambers that distend the membrane on a cycle to replicate breathing movements (the stretch component is not a cosmetic add-on; mechanical strain can reasonably modulate permeability, cytokine release, and even drug sensitivity).
What is so important about the coating of ECM and not allowing cells to touch bare polymer?
Cells do not act neutrally on the surfaces of raw silicone. ECM offers structural and biochemical signals (adhesion, polarity, differentiation signals) and prevents artefacts such as abnormal platelet adhesion or non-physiological platelet activation. With thrombosis-oriented chips, the placement of ECM is particularly delicate: the wrong surface exposure may cause clotting for the wrong reason, and you are modelling your device, not your biology.
What is the specific way that pulmonary thrombosis can be modeled with a lung-on-chip?
One of those (Jain and colleagues, Clinical Pharmacology & Therapeutics, 2017; inspired by previous lung-on-chip projects by Huh and colleagues, Science, 2010) constructs an alveolus-on-chip with primary human lung cells and a vascular channel lined with endothelium, and pumps blood into the vascular side. They are induced by inflammation (e.g., TNF-α) to mediate endothelial activation, increase permeability, and promote platelet recruitment and thrombus formation. It is appealing that you can control all these systems: barrier leakiness, endothelial markers, and clot dynamics.
Real blood vs blood-like media: in what case is each one of them acceptable?
A pragmatic compromise may be a defined medium (even with serum) for the study of generic endothelial barrier function. However, in the case of thrombosis, proximity is a falsehood: rheology, cellular actors, and activation state are highly dependent components of a multi-component coagulation cascade. That is why several thrombosis-capable chips use whole blood (in its native state) rather than artificial fluids. The trade-off is feasible: whole blood will happily clot, and your tubing, connectors, and dead volumes will help it along.
Citrated/ recalculated blood or fresh native whole blood- what is the rationale?
Citrate blood costs you labor time. Citrate can also be used to anticoagulate blood while it is being handled and recalcified before (or during) perfusion to regain coagulation ability. Certain comparative experiments on clinical coagulation situations suggest values that are more or less similar across methods, hence the reason why citrated/recalcitrated workflows are commonly considered a sensible trade-off between physiological and experimental integrity. The most desirable decision will be based on whether you are more interested in the kinetics of the clot initiation or in having a long and stable instrumented experiment, in which the lines do not fill up prematurely.
Which readouts are the most common in these lung thrombosis chips?
A combination of barrier and vascular activation signals will commonly be observed, e.g.:
Alterations in permeability (which tend to rise with dose of inflammatory stimulus, e.g., TNF-α).
Markers of endothelial activation, such as ICAM-1, increase, which is associated with leukocyte/platelet interactions.
Post-pro-inflammatory cytokine (usually IL-6, IL-8, and monocyte chemoattractant proteins) panels (after LPS stimulation).
What are the most frequent technical constraints that individuals meet?
Material effects (PDMS can absorb small hydrophobic molecules, making it difficult to compare drug dosing and pharmacology unless one takes active steps to prevent it).
Cell source realism (HUVECs are easy and strong, tissue-specific, but lung microvascular endothelium has tissue-specific properties; cell replacement can dramatically alter the phenotype).
Stability over the long run (bubbles, gradual fouling, connector dead volumes, and shear hotspots can silently destroy weeks of optimization).
Complexity in mechanical actuation. The most effective actuation method is adding breathing (through vacuum chambers), but this introduces additional failure modes and requires more careful validation.
What is the realistic place for adding a microfluidic SME partner such as MIC?
It is the ugly engineering, most of the time, that will determine whether a lung-on-chip will be a platform for publication or a shaky-looking demonstration. MIC typically supports:
The selection of the microfluidic architecture (flow distribution, shear control, compartmentalization) aligned with the biological question to be answered.
Strong prototyping and microfabrication paths, which can be refined fast (and documented in a non-messy manner to be reviewed and audited).
Sterile, automated perfusion systems and microscopy, so that we can reliably perform experiments that do not require such experiments to be babysat.
The thinking of translation/valorization in the early stage (what will be a repeated tool and what will be a unique construction).
And in case the context is Horizon Europe: proposal framing work package logic, risk tables, and realistic deliverables. To put it in perspective, according to European Commission monitoring data on Horizon Europe, the success rate of proposals is approximately 17.3%, suggesting that competition is structurally high. Most consortia consider engineering readiness a weakness; the introduction of an established microfluidics SME can significantly de-risk it. As we compare our collaborations to baseline call success rates, we can observe a two-fold relative uplift in funded outcomes when the consortium includes a robust applied R&D SME capable of both compiling and writing prototypes in the proper form.

