Molecular Transport Platform
Advanced platform for controlled molecular transport studies
Automated platform
Reduced hands-on time for extended studies
Independently regulated microenvironments
Customize parameters on either side of the barrier
Dynamic results over time
Characterize molecular transport kinetics

Need a microfluidic SME partner for your Horizon Europe project?
Exploring complex interactions with barrier models
Our new microfluidic platform is designed to help researchers study molecular transport across different barriers in the body, such as the blood-brain barrier, placenta and gut. This kind of research is becoming more popular because it allows scientists to understand complex interactions between molecules and cells in a controlled way. By focusing on molecular transport, researchers can gain deeper insights into how substances move through these barriers [1].
However, these experiments can be tricky to set up and reproduce in different labs. To solve this problem, our team created a system that automates the key parts of molecular transport experiments. This makes it easier for researchers to get consistent results and study how molecules move in different environments, which is crucial for advancing our understanding of molecular transport [2].
Platform Setup
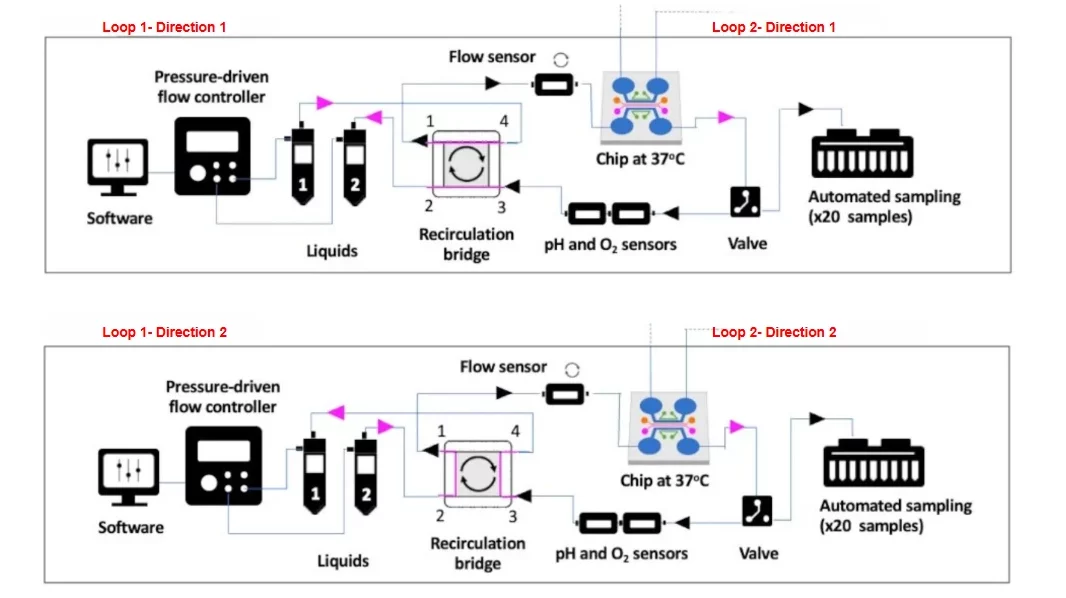
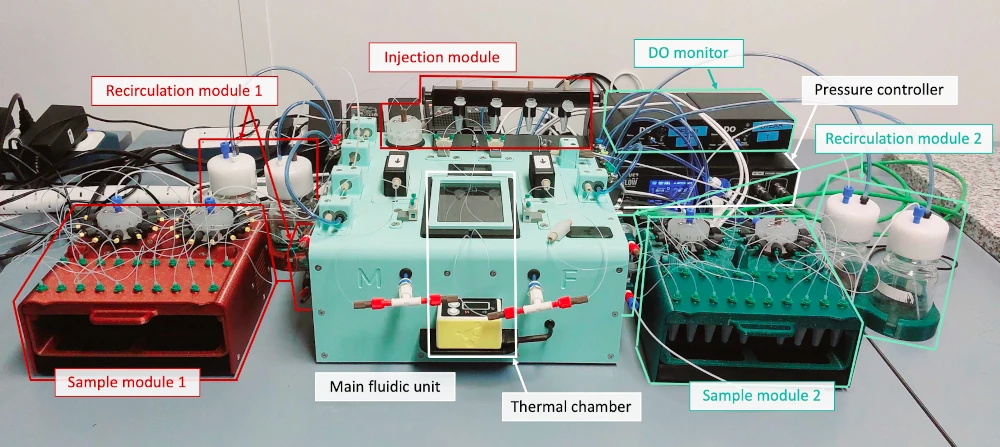
Our molecular transport platform is designed with two sides, each capable of independent control and monitoring.
Each side includes:
- A recirculation system to continuously perfuse the cells or microenvironment with a fail-safe mechanism to prevent clogging.
- A temperature controller to maintain a stable environment.
- O2 sensors to monitor the microenvironment.
- A sample collector for automated, time-resolved collection.
- Compatibility with various cross-membrane chips to connect both sides.
The functionalities are centrally controlled via a dedicated software interface.
References
Image:
Batista, P. H. J., & Quilles Jr., J. C. (2020). Drug Metabolites: General Features and Most Applicable Analytical Methods of Studies. [Journal Name].
Text:
Maoz, B. M., et al. (2018). A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nature Biotechnology.
Park, T. E., et al. (2019). Hypoxia-enhanced blood-brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nature Communications
Compatibility and applications
- Drug permeability studies
Studying the permeation of drugs across cell monolayers. - Toxicology assays
Evaluating the impact of toxic substances in vitro. - Cancer research
Analyzing the transport of cancer cells through the endothelial barrier. - Nutrient transport
Mimicking the transport of nutrients into artificial tissues. - General cell culture
Perfuse the cells continuously, with a fail-safe mechanism in case of clogging - And many more!
Molecular transport platform technical specifications
| Characteristics | Specifications |
|---|---|
| Accuracy | +/- 2.5 mbar |
| Flow rates | 0-5ml/min depending on flow sensors |
| Air consumption | few ml/min |
| Response time | 140 ms |
| Settling time | 2750 ms |
| Overshoot | 140 ms |
| Recirculation Bridge | Internal volume: 4 ml/bridge |
Automated sampler
| Characteristics | Specifications |
|---|---|
| Number of samples | Up to 20 samples per side |
| Volume of collection vial | 1,5 to 2 ml Eppendorfs |
O2 sensors
| Components | Technical specifications |
|---|---|
| Wetted Material | PTFE |
| Dimensions | 10x10x10 cm (control unit)
3x1x1 cm (sensing unit) |
| Admissible Flow rates | 1-100 µL/min |
| Accessible Oxygen Levels | 0-20 %DO |
| Stability of the control | +/- 0.5 %DO/td> |
| Dynamic range of control | 0.5% DO / min |
| pH range | 6-8 pH |
| Stability of the control | +/- 0.5 pH |
Frequently asked questions
How many chips can we run at the same time?
Currently, the platform can hold only one cross-membrane chip.
What are the characteristics of the membrane of the chip?
The platform was designed to host most types of chip, whether commercial or home-made, with the right adapters. So, the membrane technical specifications depend on your choice of material.
Can the platform be placed inside a CO2 incubator?
No, the platform was designed to be independent of the incubator.
Do I need to use a specific chip?
No, the platform was designed to host most chips, whether commercial or home-made, with the right adapters.
Do you provide the chips with the cells already grown inside?
No, we provide the fluidic circuit and automation to run various barrier model experiments with this platform. The biological part is out of our scope. You will need to develop and culture your cells yourself, and we can help you integrate your biological model into the platform.
How can we help your experiment?
This pack is in beta testing phase. So, although the instruments are not fully industrialized, we can provide extensive support as part of our beta testing program. Get in touch to see if you are eligible.




Funding and Support
The LIFESAVER and Micro4Nano projects helped develop this instrument. These projects are funded by the European Union’s H2020-LC-GD-2020-3, grant agreement no. 101036702 (LIFESAVER) and H2020-MSCA-RISE-2020, grant agreement no. 101007804 (Micro4Nano).
Products & Associated Accessories
FAQ - Molecular transport platform
What is the Molecular Transport Platform, in non-technical terms?
It is a two-compartment, automated microfluidic system that is meant to measure the crossing of a biological barrier by molecules (or even cells) at controlled flow. The key principle is to operate two sides of the barrier independently (media, oxygenation, temperature, sampling), and measure time dependent transport kinetics, as opposed to an endpoint measurement.
What is it, specifically, that is automated here, what do I cease doing manually?
Some of the mind-numbing repetitive tasks are shifted into an instrumented loop: constant perfusion through recirculation (with a safety measure that aims to minimize cases of clogging) and temperature regulation, oxygen measurements, and automatic sample sampling at regular intervals. You still write the biological model, but you are not humping tubing that you get to put it on the biological model any more, you are not scheduling the collection time by the phone alarm, you are not attempting to recreate a perfect manual workflow on multi-hour (or longer) runs.
Am I able to manipulate both sides of the barrier separately (donor vs receiver, hypoxia vs normoxia, etc.)?
Yes–each side has its own independent control/monitoring which is one of the key points that can be sold. Theoretically it is designed to work under asymmetric conditions: varying oxygen concentrations, varying media formulations, varying dosage regimes. This is usually the difference between a good demo and one that you can sell in a consortium deliverable.
What is the number of chips that I will be able to run concurrently?
At this point: a single cross-membrane chip at a time on the platform. It would be a restriction, but it is a design trade of a goal of high-quality kinetics with close control (not as many chips as possible with a lot of drift).
Is there a certain type of chip or a type of membrane that I have to use?
No, at least not in principle. The site is made to support the majority of cross-membrane chips (commercial or home-made) with the necessary adapters, meaning that the membrane specifications depend on the chip you select (material, pore size, thickness, coating strategy, and so on). That flexibility is important when your consortium has already a preferred chip format, or when you must conform to an established barrier protocol of one of the labs.
What are the main pressure/flow performance figures?
The listing of pressure accuracy of +-2.5 mbar, flow rates of 0-5 mL/min depending on the flow sensors selected and a response time of 140 ms (settling time 2750 ms). Air consumption is characterized as a couple of mL/min. The volume of the recirculation bridge provided is 4 mL per bridge.
What is the way that sampling works – how many timepoints can I get without cracking the system?
The automated sampler is designed with a maximum of 20 samples on one side with Eppendorf 1.5-2 mL collection vials. In the case of transport kinetics, you can normally get away with that “20 per side” and not only get fast-time transients but also subsequent steady-state regimes without reducing your experiment to a manual control test.
Oxygen control, range, stability, what do we mean?
O2 sensing/control module is requested to be used in the accessible oxygen of 0-20% DO, the control stability of +-0.5% DO, and the dynamic control range of approximately 0.5% DO per minute. Listed admissible flow rates are 1-100 uL/min with a wetted material being PTFE. (A pH window of 6-8 is also observed with a stability of +-0.5 pH.)
Is the entire platform capable of being put in a CO2 incubator?
No, the platform is made to be independent of an incubator. In practice, this normally implies that you use the temperature control and microclimate of the platform, and not the CO2/temperature envelope of the incubator.
Do you sell cell-pre-grown chips (ready-to-run biology)?
No, the biological production pipeline is not it, it is the scope of the fluidic circuit + automation to run barrier-model experiments. You grow and inculcate your own cells; MIC can aid you to bring your biological model to the platform so the biology and fluidics cease conflict with one another (where many good ideas quietly fail, sadly, to materialize).
We are developing a Horizon Europe proposal: what is the fit of this platform (and MIC) into an acceptable work package?
There is an increasing trend in the use of barrier models that fill the gap between in vitro work, which is too simple and too cheap/fast to provide useful information, and the complex work in vivo, which is costly and time-consuming, with support of mechanistic questions on transport that a reviewer can interpret as real science. Even the platform itself was created, with the assistance of EU-funded initiatives (e.g. LIFESAVER grant agreement no. 101036702; Micro4Nano grant agreement no. 101007804), which come in handy when you have to justify maturity and other prior validation pathways in a proposal. Beyond the instrument, introducing a microfluidic SME into the consortium generally enhances implementation and risk management: someone must own the implementation, integration, and prototype iteration loop, and universities often simply do not have the bandwidth to do that once the project is in progress.

