Droplet breakup pack
Controlled and spontaneous droplet splitting in a microfluidic chip
Passive droplet breakup
No complex active splitting geometries
Ideal for artificial life models
Hosts functional molecular complexes at the water-oil interface
Tunable breakup timing
Controlled by flow rate and surfactant concentrations
Intrinsic chemical mechanism
Driven by non-equilibrium surfactant dynamics

Need a microfluidic SME partner for your Horizon Europe project?
The representative animation of droplet division in a microfluidic channel was generated using Gemini’s Nano Banana Pro.
Microfluidic Spontaneous Droplet breakup
Achieving droplet splitting in microfluidics has historically required complex channel geometries, such as T-junctions with obstacles, or external active forces. Now, you can achieve spontaneous splitting of water-in-oil emulsion droplets using the Droplet breakup Pack!
Based on the principle of non-equilibrium catanionic surfactant systems [1], this all-in-one solution allows researchers to generate droplets that spontaneously divide into two or more daughter droplets after a specific lag time.
Unlike the static oil-in-water experiments described in early literature [2,3], this pack is optimized for water-in-oil (W/O) emulsions in a continuous flow microfluidic chip. By injecting an anionic surfactant solution downstream of the droplet generation, the system triggers a transient instability [1] that pulls the droplet apart.
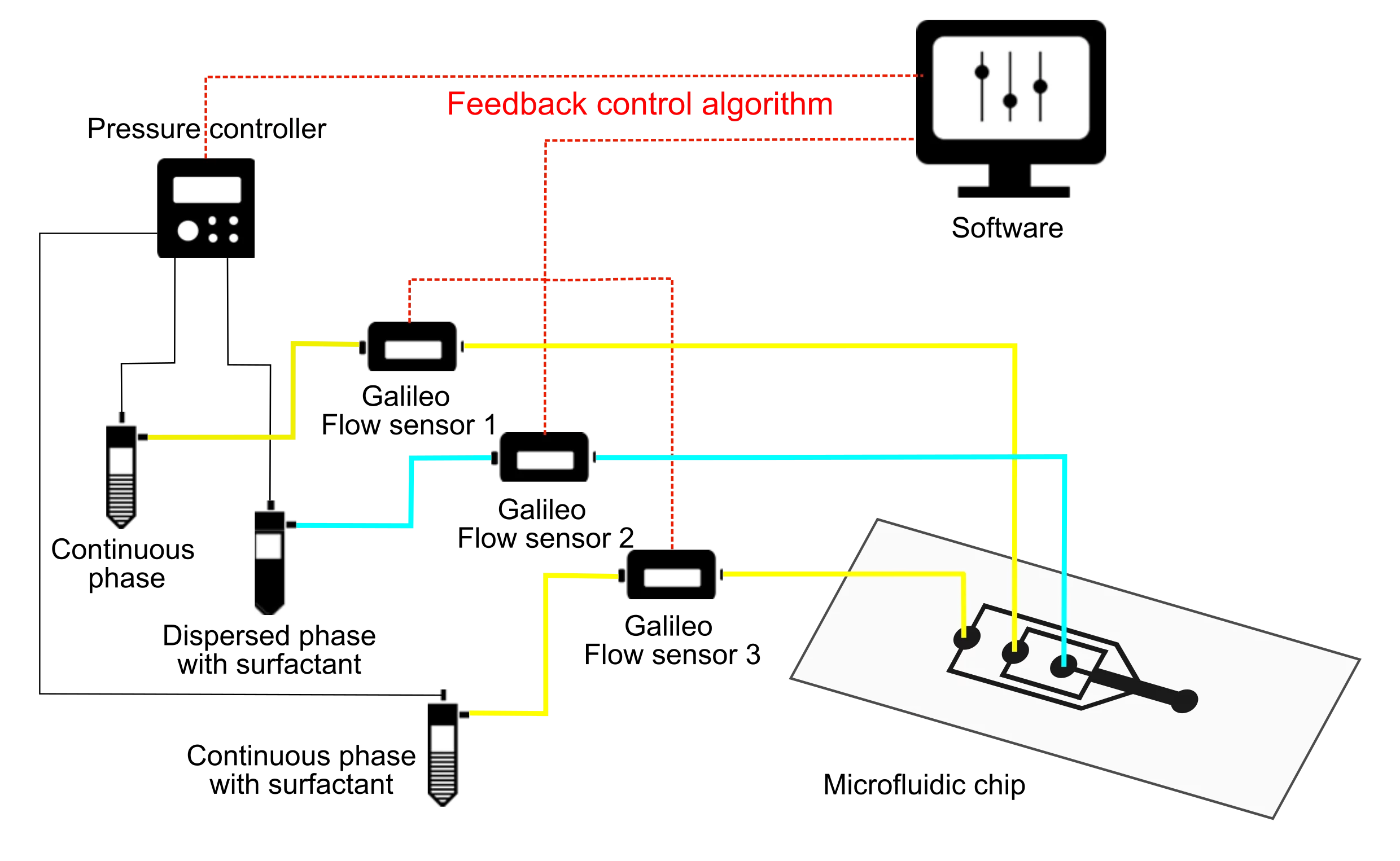
A standard droplet breakup pack contains two pumping channels to push the two fluids to generate water-in-oil droplets in a microfluidic chip. It also includes an additional pressure channel for injecting a surfactant solution downstream. Flow rates can be measured and precisely controlled, thanks to high-precision Galileo flow rate sensors.
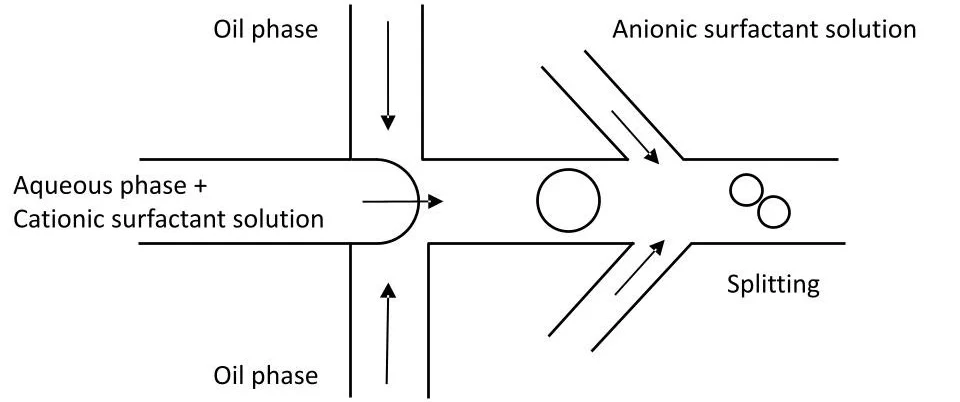
Droplet splitting setup
A preassembled pack guarantees compatibility between the chemical reagents and the microfluidic hardware. It is piloted by our flexible software, which precisely controls the flow rates, thereby controlling the timing of the division event.
The Droplet breakup pack contains:
High precision pressure pump
3x Galileo flow sensor
Software (Galileo user interface for flow stability)
Droplet splitting Chip (Flow-focusing junction with downstream injection port)
Chemical Starter Kit
The chemical starter kit can be:
- Cationic surfactant (e.g., CTAB) for the dispersed aqueous phase
- Anionic surfactant (e.g., Fatty acid/Oleate) for the trigger solution
All necessary accessories: Tubing, connectors, filters
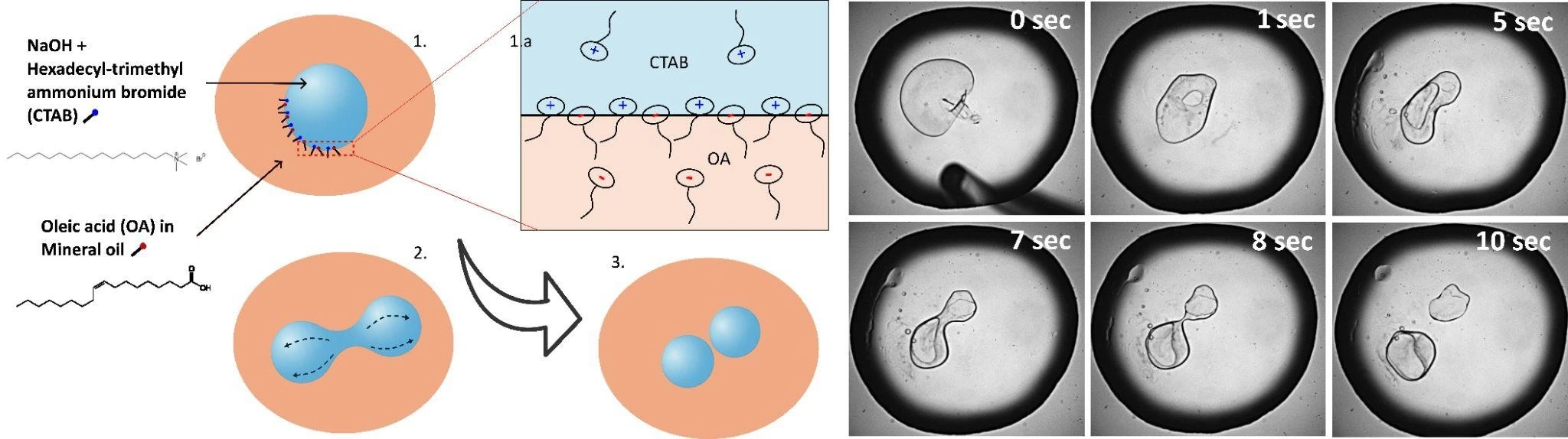
Spontaneous Droplet splitting Principle
The spontaneous splitting utilized in this pack is driven by a transient decrease in interfacial tension in a catanionic surfactant system.
- Droplet Generation: Aqueous droplets containing a cationic surfactant are generated in an oil phase using a standard flow-focusing junction.
- Trigger Injection: Downstream, a solution containing an anionic surfactant is introduced into the channel.
- Non-Equilibrium Instability: As the anionic surfactant diffuses to the droplet interface, it pairs with the cationic surfactant. This momentarily lowers the interfacial tension to near-zero values.
- Shape Change & breakup: The droplet destabilizes, elongating into a toroidal or dumbbell shape due to internal Marangoni flows, and eventually breaks into smaller daughter droplets.
- Stabilization: As the system approaches equilibrium, the interfacial tension rises again, stabilizing the newly formed daughter droplets.
This process mimics rudimentary cell division, making it a powerful tool for bottom-up synthetic biology and “artificial life” research [4].
Customize your pack
The Droplet splitting chip and chemical kits are fully customizable to suit your specific “artificial life” [2] or emulsion splitting experiments.
For Water-in-Oil (W/O) droplets, surface properties are critical to prevent wetting.
- Hydrophobic Glass: Standard for robust chemical resistance, especially when using organic solvents like nitrobenzene.
- Fluorophilic Polymer: Recommended if using fluorinated oils for high-stability biological encapsulation.
- Channel Dimensions: Select nozzle sizes from 10 µm to 100 µm to define your initial parent droplet volume.
Surfactant starter kits:
The number of daughter droplets generated per breakup event depends strictly on the surfactant pair and concentration ratios used [1]. We offer pre-optimized catanionic pairs:
- Binary Fission Kit (CTAB / Decanoate): Optimized to split one parent droplet into two equal daughter droplets.
- Multiple Fission Kit (CTAB / Oleate): Generates three or more daughter droplets on average.
- Burst Fission Kit (DTAB / Oleate): Creates a “burst” of ten or more micro-droplets for massive parallelization.
Reaction Control Modules:
- Delay Lines: We can integrate serpentine channels of varying lengths to precisely tune the “lag time” (typically 5–10 seconds) between droplet generation and the spontaneous breakup event.
- Downstream Injection: Add a second injection port to introduce the trigger surfactant after droplet formation, ensuring splitting occurs exactly where you want it in the channel.
You can contact our researchers to answer any questions about this droplet breakup pack and how it can match your specifications.
Frequently asked questions
Can I control the number of daughter droplets?
Yes. Research shows that specific surfactant pairs dictate the breakup behavior. For example, pairing CTAB (in the aqueous phase) with Decanoate (in the oil phase) typically yields two daughter droplets, whereas using DTAB yields over ten [1]. Our experts can help you select the right pair for your desired output.
Will the droplets fuse back together after splitting?
No. Once the breakup event is complete, the system approaches equilibrium and the interfacial tension rises again, stabilizing the daughter droplets [1]. They remain separate and stable, making them suitable for further downstream analysis or collection.
Why use this instead of a standard droplet splitter?
Standard splitters require precise alignment and high flow rates to mechanically break droplets. Spontaneous fission is “intrinsic”—it happens automatically due to the chemical composition. This is particularly useful for modeling biological cell division, studying non-equilibrium thermodynamics, or creating self-replicating chemical systems.
Is this compatible with biological samples?
While the original research utilized nitrobenzene (an organic oil) [1], our pack can be adapted for biocompatible oils. However, please note that the “breakup” mechanism relies on specific ionic surfactant interactions, so compatibility with sensitive enzymes or cells should be tested.
Can a pack be customized based on my specific application?
Yes! Our experts will establish which instruments are best suited for your application, such as the type of flow sensor or the number of flow controller channels you need to perform your experiment. Drop us a line at innovation@microfluidic.fr.
Reference
- Caschera, Filippo, Steen Rasmussen, and Martin M. Hanczyc. “An oil droplet division-fusion cycle.” ChemPlusChem 78.1 (2013): 52.
- Sumino, Yutaka, et al. “Spontaneous deformation of an oil droplet induced by the cooperative transport of cationic and anionic surfactants through the interface.” The Journal of Physical Chemistry B 113.48 (2009): 15709-15714.
- Okada, Masahide, et al. “Spontaneous deformation and fission of oil droplets on an aqueous surfactant solution.” Phys. Rev. E (2020).
- Zwicker, David, et al. “Growth and division of active droplets provides a model for protocells.” Nature Physics 13.4 (2017): 408-413.



Funding and Support
This project has received funding from the European Union’s Horizon research and innovation program under the Marie Skłodowska-Curie grant agreement no. 101119956 (DarChemDN).
Products & Associated Accessories
FAQ - Droplet breakup pack
1) What is it, precisely, the Droplet Breakup Pack?
It is a plug-and-play microfluidic device that is meant to create water-in-oil droplets and then cause them to spontaneously divide into droplets after a programmable delay period. The thing is that the breakup is non-energetic: you do not require a certain electric field, acoustics, a laser, a mechanically aggressive splitter geometry, etc. to make division occur.
2) What is the difference between this and the standard droplet splitters (T-junction splitters, obstacle splitters etc.)?
This type of splitter is largely geometric: the droplets are forced into a constriction or branching network that is designed to split them; and they are separated by hydrodynamic stress. That is working, though it can be sensitive (alignment, narrow operation window, increased flow rates, and in some cases, weak stability).
In this case, the division is chemically induced and intrinsic: the droplet separates due to the fact that the interfacial conditions are taken out of equilibrium- the chip does not require a special splitting architecture.
3) How is it possible to split the droplets by a mechanism? (In plain technical terms.)
The pack is based on non equilibrium catanionic surfactant system. In practice:
-A cationic surfactant is used to form droplets in the aqueous phase.
-A trigger is an anionic surfactant that is injected downstream.
At the point that the anionic surfactant arrives at the interface and matches with the cationic one, the interfacial tension momentarily goes to zero (the page specifically explains that the interfacial tension becomes nearly zero).
This temporary condition creates internal flows that are vigorous due to Marangoni effects, the droplet becomes (usually toroidal/dumbbell-shaped) as a result of the effect, and then the droplet fractures into daughters.
After stabilizing, the interfacial tension increases once more and the new droplets stabilize.
4) Am I able to tune the droplet separation?
Yes. Timing may be adjusted through flow rates and surfactant concentrations and the pack may incorporate serpentine delay lines to give focus on a desired time window. The page states that the standard range of lag time is approximately 5-10 seconds (not a law, but merely a design objective).
5) Does the level of daughter droplets (2 vs 3+ vs “burst” fragmentation) depend on me?
Yes, to a great large extent, by choice of the surfactant pair and ratios. The pack description provides specific modes:
-Binary fission (examples: CTAB / Decanoate) – there are usually 2 daughters.
-Multiple fission (example: CTAB / Oleate)- 3 or more daughters on average.
-Burst fission (such as DTAB / Oleate) – regularly 10 or more micro-droplets.
6) Does the daughter droplets re-fuse at a later stage?
At the planned working conditions, no: once the interfacial unsteadiness is over and interfacial tension is restored, the system will stabilize with the droplets on the separate phase. This method is desirable to downstream route, image, or collect with that stability as being one such reason.
7) What is really contained within the pack (hardware + consumables)?
A typical set up as stated on the page comprises:
-A pressure pump of high accuracy.
-3 Galileo flow sensors (in case of tight flow stability control and measurement)
-Galileo user interface control software
-A droplet separating chip (flow focusing droplet generator + downstream injection port)
-A chemical starter kit (one of the following examples has been used: CTAB as cationic surfactant; oleate / fatty acid as anionic trigger)
-Tubing/connectors/filters and similar useful accessories.
8) What does it mean by customizing something when my experiment is not standard?
A good deal, and the page is disproportionately graphic:
-Chip material & surface characteristics: chemical the hydrophobic glass is used to maintain chip chemical durability (even mentions solvent resistance, using nitrobenzene as an example), or fluorophilic polymer in case you are using fluorinated oils to ensure stable encapsulation.
-Channel dimensions: can be chosen nozzle sizes, 10 um to 100 um, and, in effect, the parent droplet volume scale.
-Fluidic architecture: optional additional injection ports, delay lines and other where and when auxiliary controls to cause breakup.
9) Can it be used with biological samples (cells, enzymes, delicate complexes)?
Potentially, but it is not automatically biocompatible, that is, per se, and that is a point. The disaggregating process is based on ionic surfactant contacts, a process that may be severe to sensitive biology. The original research setting mentioned on the page was with organic oil (nitrobenzene), although they also mention that the pack can be modified for more biocompatible oils. In practice, a brief compatibility screen (viability/activity, adsorption, leakage, interfacial effects) would be expected.
10) What makes MIC present this pack as ideal for artificial life / protocell-style models?
Due to the similarity of the dynamics to a minimalist growth-and-division story: interfacial-tension collapse, shape change through self-organization, and fission, all without an external agent. It specifically describes this as a bottom-up synthetic biology / artificial life modeling tool.

