Artificial Cell Droplet Microfluidic pack
Plug-and-play pack for artificial cell formation using microfluidic droplets
Great monodispersity and reproducibility
Better control than other artificial cell synthetizing methods
Automatized production
Study the structure and function of living cells for novel therapeutics development
Monodisperse and unilamellar vesicles
Bottom-up approach to build biomimetic structures

Need a microfluidic SME partner for your Horizon Europe project?
Artificial cell synthesis production principle picture by AI et al. (2019), artificial and natural cell comparison by Trantidou et al. (2017), liposome production with octanol by Deshpande et al. (2016)
A pack for artificial cells, protocells, and GUVs production
Creating artificial cells is a trending topic for researchers studying the origin of life or manufacturing synthetic building blocks for prospective applications like diagnostics or drug delivery.
Microfluidics allows better reproducibility, monodispersity, vesicle size control, encapsulation efficiency, and membrane homogeneity, making it the most efficient method for creating artificial cells.
Our researchers assembled a microfluidic platform to obtain vesicles that mimic cell functions for researchers and industries that want to transition to microfluidic technology. Artificial cells are more accessible to analyze and control and can be designed with specific variables and parameters that are more easily maintained than natural ones.
This pack can be personalized to produce artificial cells, including giant unilamellar vesicles (GUVs) or simple/double emulsions. It is also perfect for obtaining monodisperse and stable biomimetic cell-like bilayer structures. Our experts will help you determine your needs depending on your application and provide customer support to fulfill your experiment goals.
The pack includes a flow controller with flow sensors for continuous flow rate control. You can combine this controller with your homemade microfluidic chip or with a double emulsion microfluidic chip from ChipShop. Microfluidic chips are transparent and can be easily combined with microscopy.
A pressure-driven flow controller provides stable and precise flow control, compared to syringes and peristaltic pumps.
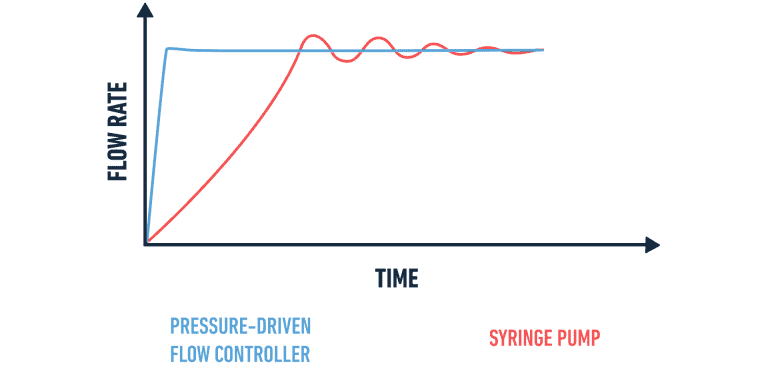
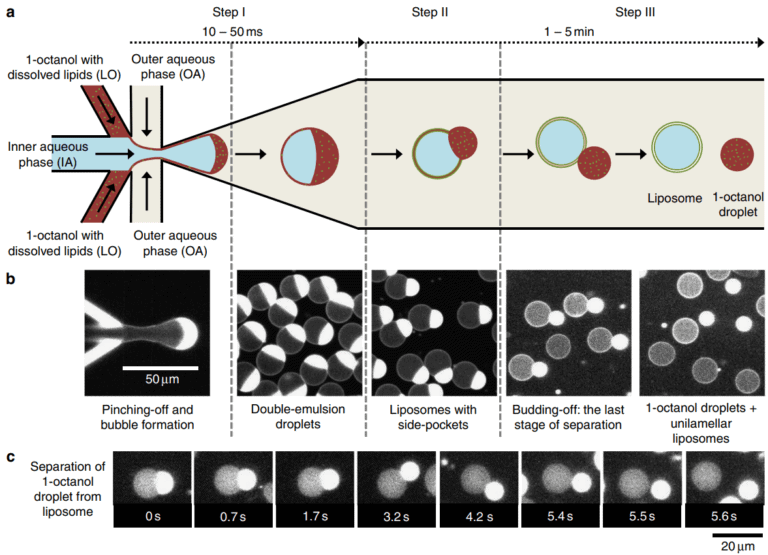
The production of the double emulsion at the base of this particular artificial cell synthesis method is a well-studied application in microfluidics because of its advantages compared to bulk techniques.
The artificial cell is formed thanks to two junctions inside the microfluidic chip that allow water-in-oil-in-water (WOW) monolayered droplets to be produced. Water-in-oil (WO) droplets could already be considered artificial cells, but oil as a continuous phase lacks biocompatibility. It is possible to use a homemade microfluidic chip, usually in Polydimethylsiloxane (PDMS), for double emulsion droplet production or a droplet generator chip from microfluidic ChipShop (fluidic 1480).
A critical step in obtaining artificial cells is to properly functionalize the microfluidic chip so that it has a first hydrophobic junction and a second hydrophilic junction by changing the material’s surface properties.
Artificial cell droplet pack setup
The pack contains several instruments that can form the following platform. Each instrument is compatible with the others, piloted by the same software and comes with a dedicated user guide for beginner friendly step-by-step setup.
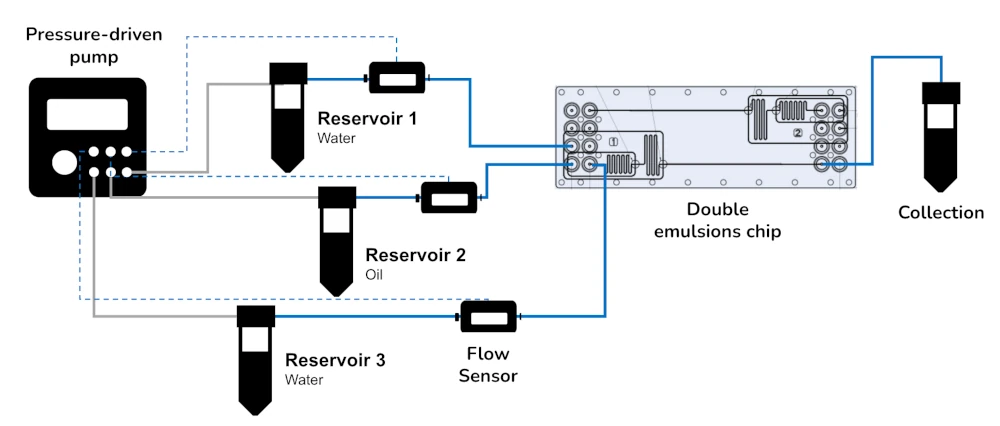
The artificial cell droplet pack contains:
Flow sensor (Galileo, MIC)
Software (Galileo user interface)
Flow controller
Fittings, tubings & luers
Reservoirs
User guides for instruments
Can contain the fluidic 1480 microfluidic chip from microfluidic ChipShop
Microfluidic artificial cells applications
Using lipid vesicles to produce protocells to study the origin of life or to produce artificial cells that will mimic some functions of natural cells to investigate their properties and dynamic behaviors is a recent, trending and very dynamic research subject in the past few years. The full complexity of a natural cell has still yet to be fully mimicked with a bottom-up method [1].
The challenge is to reproduce the cell membrane that contains receptors that communicate, move, and sense their local environment. The inner part of the cell contains genetic material and enzymes responsible for cellular processes such as replication, protein synthesis and metabolism or growth-related processes [2].
There are several ways to produce artificial cells using microfluidics. Giant unilamellar vesicles (GUVs) are capsules formed by lipid bilayer membranes (or liposomes) bigger than 10 µm in diameter that present high similarity with cellular membrane and can be used as compartments to create artificial cells [3]. It has been shown that GUVs can be used to encapsulate proteins, DNA and RNA [4].
Microfluidics is the best method for producing artificial cells with improved vesicle size consistency, membrane homogeneity, encapsulation efficiency, and throughput compared to batch methods. Several methods have been used to produce GUVs inside a microfluidic chip with double emulsions [5-8].
Proteins can be incorporated to giant unicellular vesicles bilayered membranes to mimic cellular functions including the development of diseases, metabolism, and homeostasis [9]. The composition of the membrane can be tuned by changing the composition of the lipids or the lipid mixtures used to produce the artificial cells [10].
Artificial cells can be stable for more than a month [11-12].
Using microfluidics is also very useful for the creation of artificial organelles in artificial cells [13-14].
References
1. Walde, P. (2010), Building artificial cells and protocell models: Experimental approaches with lipid vesicles. Bioessays, 32: 296-303.
2. Martino Chiara and deMello Andrew J. 2016 Droplet-based microfluidics for artificial cell generation: a brief review Interface Focus.
3. Sato, Y.; Takinoue, M. Creation of Artificial Cell-Like Structures Promoted by Microfluidics Technologies. Micromachines 2019, 10, 216.
4. Yu B, Lee RJ, Lee LJ. 2009 Microfluidic methods for production of liposomes. Methods Enzymol. 465, 129–141.
5. Petit, Julien, et al. “Vesicles-on-a-chip: A universal microfluidic platform for the assembly of liposomes and polymersomes.” The European Physical Journal E 39.6 (2016): 1-6.
6. Deshpande, S.; Caspi, Y.; Meijering, A.E.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 10447.
7. Arriaga, Laura R., et al. “Ultrathin shell double emulsion templated giant unilamellar lipid vesicles with controlled microdomain formation.” small 10.5 (2014): 950-956.
8. Van Swaay D, deMello A. 2013Microfluidic methods for forming liposomes. Lab Chip 13, 752–767.
9. Kamiya, K. Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology. Micromachines 2020, 11, 559
10. M. Komiya, M. Kato, D. Tadaki, T. Ma, H. Yamamoto, R. Tero, Y. Tozawa, M. Niwano, A. Hirano-Iwata, Chem. Rec. 2020, 20, 730.
11. Osaki, Toshihisa, and Shoji Takeuchi. “Artificial cell membrane systems for biosensing applications.” Analytical chemistry 89.1 (2017): 216-231.
12. Martino, Chiara, et al. “Protein expression, aggregation, and triggered release from polymersomes as artificial cell‐like structures.” Angewandte Chemie 124.26 (2012): 6522-6526.
13. Masamune Morita, Dr, Kaoru Katoh, and Naohiro Noda. “Direct observation of bacterial growth in giant unilamellar vesicles: a novel tool for bacterial cultures.” ChemistryOpen 7.11 (2018): 845.
14. Yamashita, Hitoyoshi, et al. “Generation of monodisperse cell-sized microdroplets using a centrifuge-based axisymmetric co-flowing microfluidic device.” Journal of bioscience and bioengineering 119.4 (2015): 492-495.
15. Deshpande, S., Caspi, Y., Meijering, A. et al. Octanol-assisted liposome assembly on chip. Nat Commun 7, 10447 (2016).
Customize your pack
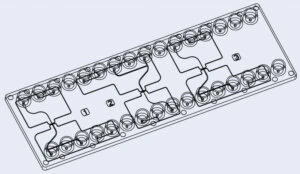
The fluidic 1032 droplet generator chip from microfluidic ChipShop contains three parts, each containing two junctions close together that can be used to produce double emulsions including GUVs. The chip is available in polycarbonate (PC) and in Cyclic Olefin Copolymer (Topas COC).
Our Packs are fully customizable to fit your needs perfectly. Our microfluidic specialists and researchers will help you choose the best instruments and accessories. They will accompany the setup of your microfluidic platform until you obtain your first experimental results.
We assembled other packs for cell biology like the cell size sorting and the cell aligner packs.
Contact our experts to answer any questions about this artificial cell droplet microfluidic pack and how it can match your specifications!
Frequently asked questions
How can we help your experiment?
This pack is in beta testing phase. So, although the instruments are not fully industrialized, we can provide extensive support as part of our beta testing program. Get in touch to see if you are eligible.
Can a pack be customized?
Yes! Our experts will establish which instruments are best suited for your application, such as the type of flow sensor or the number of flow controller channels you need to perform your experiment. Contact us using the “talk to our experts” green button above.
Can I buy individual instruments?
Our instruments are in beta testing phase and can be tested as a pack or individually, so get in contact with our team to know how our beta testing program works.



