Drug testing: organ-on-chip models vs. standard models
Author
Alessandra Dellaquila, PhD
Publication Date
Keywords
Drug Testing
disease modeling
Organ-on-a-chip
Animal models
Cell cultures
tissue engineering

Need advice for your organ-on-chip model?
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Introduction to organ-on-chip models
In vitro and in vivo models are widely used to investigate human pathophysiology and toxicology studies. The goal of this review is to highlight the main advantages and limitations of each model and to show how the use of organ-on-chip model technology can address their drawbacks.

The article refers specifically to lung systems (i.e., capillary-alveolar interface). Still, it also applies to other organs since the characteristics of joint in vivo/ in vitro systems result in being related to the system itself rather than the organ/ tissue studied.
You can have a look at this review for a description of the use of the alveolar-capillary barrier to design microfluidic lung-on-a-chip systems. To start using microfluidics to build lung-on-a-chip models, check out our pack.
You can continue reading this review to learn more about the origin and design of lung-on-a-chip systems.
Overview of organ-on-chip in vitro and in vivo models
Organ-on-chip models vs. ANIMAL MODELS [1, 2]
| |
+ |
|
– |
|
Organ-on-chip models vs. EX VIVO CULTURES (e.g., biopsy samples) [3, 4]
| |
+ |
|
– |
|
Organ-on-chip models vs. 2D CELL CULTURES [3, 4]
| |
+ |
|
– |
|
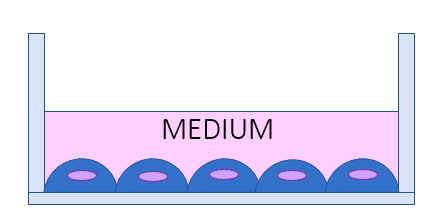
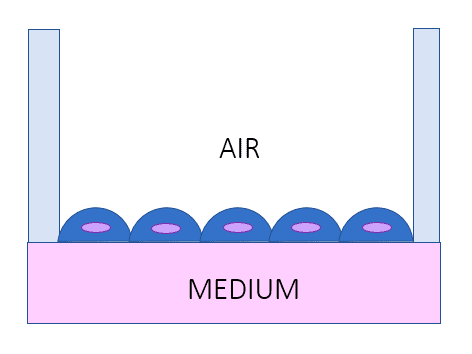
Organ-on-chip models vs. AIR-LIQUID INTERFACE LUNG MODEL [1, 5]
| |
+ |
|
– |
|
Organ-on-chip models vs. ORGANOIDS [6, 7]
| |
+ |
|
– |
|
Organ-on-chip models vs. TISSUE ENGINEERING (1)
Artificial constructs [8-10] | |
+ |
|
– |
|
Organ-on-chip models vs. TISSUE ENGINEERING (2)
Biological (decellularized) constructs [8–10] | |
+ |
|
– |
|
Organ-on-chip models as solutions for the limitations of traditional in vitro and in vivo systems
Advantages resulting from the fabrication process
- Modularity and variety of techniques/ materials for microfabrication
- The chip design can be easily created and modified based on need
- High reproducibility of fabrication protocols
- Possibility to integrate sensors for high-throughput analyses
- Possibility to develop personalized models
Advantages resulting from a microscale flow
- Mimic physiological/diseased mechanical cues at a cellular/ tissue level
- Create a controlled and dynamic microenvironment
- Recreate the air-liquid interface (specifically for lungs)
Advantages for the cellular/ biological component
- Create a complex tissue-like environment with several cell types
- Create modular devices mimicking different organ areas (ex, bronchi, alveoli, etc.…in the case of lungs)
- Reproducing physiological and pathological conditions
Advantages for time/cost of research
- Fast fabrication processes (soft lithography, rapid prototyping, …)
- Possibility to rapidly test drugs/compounds and have high-throughput outcomes.
- Possibility to easily mimic disease conditions without the need to prepare, maintain, and use animal models.

Review done thanks to the support of the DELIVER H2020-MSCA-ITN-2017-Action “Innovative Training Networks.”
Grant agreement number: 766181
Written by Alessandra Dellaquila, PhD
Contact:
Partnership[at]microfluidic.fr

References
- A. J. Miller and J. R. Spence, “In vitro models to study human lung development, disease and homeostasis,” Physiology, vol. 32, no. 3, pp. 246–260, 2017.
- B. A. Hassell et al., “Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro,” Cell Rep., vol. 21, no. 2, pp. 508–516, 2017.
- C. Blume and D. E. Davies, “In vitro and ex vivo models of human asthma,” Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV, vol. 84, no. 2, pp. 394–400, Jun. 2013.
- H. Behrsing et al., “Assessment of in vitro COPD models for tobacco regulatory science: Workshop proceedings, conclusions and paths forward for in vitro model use,” Altern Lab Anim, vol. 44, no. 2, pp. 129–166, 2016.
- R. Bhowmick and H. Gappa-Fahlenkamp, “Cells and Culture Systems Used to Model the Small Airway Epithelium,” Lung, vol. 194, no. 3, pp. 419–428, Jun. 2016.
- K. Gkatzis, S. Taghizadeh, D. Huh, D. Y. R. Stainier, and S. Bellusci, “Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease,” Eur. Respir. J., vol. 52, no. 5, Nov. 2018.
- M. Huch, J. A. Knoblich, M. P. Lutolf, and A. Martinez-Arias, “The hope and the hype of organoid research,” Development, vol. 144, no. 6, pp. 938–941, Mar. 2017.
- A. Doryab, G. Amoabediny, and A. Salehi-Najafabadi, “Advances in pulmonary therapy and drug development: Lung tissue engineering to lung-on-a-chip,” Biotechnol. Adv., vol. 34, no. 5, pp. 588–596.
- R. Langer and J. Vacanti, “Advances in tissue engineering,” J. Pediatr. Surg., vol. 51, no. 1, pp. 8–12, Jan. 2016.
- D. M. Hoganson, E. K. Bassett, and J. P. Vacanti, “Lung tissue engineering,” Front. Biosci. Landmark Ed., vol. 19, pp. 1227–1239, Jun. 2014.
Check the other Reviews
FAQ - Drug testing: organ-on-chip models vs. standard models
What are organ-on-chip models?
Organ-on-chip models are microfluidic systems that simulate the physiological microenvironment of human organs at the cellular level. They include living cells, dynamic flow conditions, and mechanical cues in order to replicate organ function. These systems fill the gap between conventional in vitro cell cultures and animal models by providing a controlled, biomimetic environment for studying human pathophysiology and drug responses.
What are the primary drawbacks of the use of animal models in drug testing?
There are some important limitations of animal models: they do not match humans in the anatomy of the lungs, cell morphology, and localization; they give an incomplete picture of the disease features; findings made are hard to compare across laboratories and among species; and they are hard to translate to clinical trials in humans. Also, animal testing is ethical but involves high costs in terms of money and time.
Why are conventional 2D cell cultures inadequate in drug testing?
Classical 2D cell cultures are systems that do not mimic the dynamic living tissue environment. They block different cell types in co-culture, cannot simulate the lung airway interface, and do not provide the mechanical stimuli present in the body. Although straightforward and applicable to some clinical diagnoses, such as tuberculosis testing, they provide little information about the complex organ-level responses.
What are the benefits of using organ-on-chip models as compared to ex vivo cultures?
Organ-on-chip models are long-term viable and reproducible compared to ex vivo cultures of biopsy samples. Ex vivo samples maintain normal tissue structure but their viability is limited with regard to time, the barrier properties are damaged by external factors, and cross-sample variability. These limitations are overcome using organ-on-chip systems based on controlled microenvironments.
What are the differences between organ-on-chip models and organoids?
Although organoids offer a 3D microenvironment and have the potential to recap the organ formation, they lack immune and vascular components and have a low level of control of the stem cell behavior and reproducibility. Organ-on-chip models are used to solve these shortcomings by including blood vascular structures, creating a well-controlled cellular environment, and incorporating physiological parameters monitored in real-time with sensors.
What are the organ-on-chip systems fabrication benefits?
Organ-on-chip fabrication has provided modularity and multiple methods and materials, simple design development and alteration, high protocol reproducibility, and sensor integration possibilities to provide high-throughput judgments. Technology allows the creation of individual models that are specific to a single patient or the disease situation and thus will be highly applicable in research and drug development.
What is the advantage of the microscale flow in the organ-on-chip models?
Microscale flow facilitates the ability of such systems to recapitulate physiological and disease mechanical cues on cellular and tissue scales. It produces controlled and dynamic micro environments which is a close replica of the in vivo environment such as the re-creation of important interfaces such as the air-liquid interface in the lung models. This dynamism is critical to proper modeling of organ response and drug reactions.
What cellular benefits are offered by organ-on-chip models?
These models produce complex tissue-like environments using many cell types, which can allow researchers to build modular devices to recapitulate different areas of an organ. In the case of lungs, it involves bronchi and alveoli areas. The systems are able to recreate the physiological and pathological conditions that offer insights that are more applicable in understanding the mechanisms of diseases and therapeutic intervention.
Which benefits in terms of cost and time does organ-on-chip models bring?
The organ-on-chip technology is used to quickly fabricate using soft lithography and quick prototyping technology. Researchers are able to test drugs and compounds on a high-throughput basis. The cost and time involved in developing the drug and testing an illness is greatly reduced by the capability to mimic the conditions of a disease with ease, avoid the preparation of animal models, their maintenance, and usage.
What are the weaknesses of tissue engineering techniques that the organ-on-chip models aim to solve?
Standard formulations of tissue engineering, which rely on artificial or decellularized scaffolds, have difficulties in recapitulating structural and mechanical forces or autonomous production of ECM. Organ-on-chip models address these drawbacks by offering the ability to control mechanical stimulation, dynamic micro-environment and integrating many tissue-based components to build more physiologically relevant systems more suited to drug testing and disease modeling.