Gut-on-chip: keeping up with the technology
Author
Emilie Grandfils
Publication Date
March 28, 2018
Keywords
Gut-on-chip
disease modeling
Organ-on-a-chip
host-microbe interplay
Drug & nutrients metabolism

Need advice for your biomedical research?
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Introduction to the gut-on-chip
The gut fulfills many critical bodily functions. It is responsible for breaking down food into nutrients that the body can absorb and use. The transport and oral absorption of drugs and nutrients is one of the essential phases of pharmacokinetics. The gut-associated lymphoid tissue (GALT) is part of the immune system and helps protect the body from harmful bacteria, viruses, and toxins. This function is less known and would require extensive research.
In addition, the gut is involved in producing essential vitamins and compounds. For example, bacteria in the gut produce vitamin K and certain B vitamins, which are vital for blood clotting and energy metabolism.
Notably, the gut communicates with the brain through the gut-brain axis. The gut microbiome plays a significant role in producing neurotransmitters such as serotonin, often called the “feel-good” chemical, which influences mood, anxiety, and stress levels.
Today's problematic
Despite this central role, we must understand all its mechanisms to develop an effective model of this complex and significant organ. Moreover, many adverse drug effects affect the gastrointestinal tract, and many gut inflammations have unknown causes, as is the case of Crohn’s disease.
Finally, there is growing evidence of the significant impact of microbial symbionts on the intestine’s health. This characteristic is not often investigated in vitro because of the difficulties in matching growth conditions between host cells and microbes.
Nevertheless, it remains a critical parameter because of the central role of the human microbiome in intestinal health and diseases [1], and the development of an in vitro platform to study host-microbe interplay is critical.
The Microfluidics Innovation Center developed a gut-on-chip pack.
Previous solutions
In vivo tests on this organ were mainly done on animals, which raises more controversy on the ethical front and provides results that cannot always be applied to humans: a drug can be toxic for rats and not for humans, and inversely.
A previous intestinal epithelial cell culture technique in vitro used mostly static culture models, as with the Transwell chambers (Corning Inc., Lowell, MA). This 3D cell culture stepped towards “in-vivo-like” environments with porous membranes and apical/basolateral sides for the chambers. Nevertheless, it is still missing crucial characteristics of the intestinal tissue to be a good predictive model of the intestine.
The gut-on-chip solution
Given the complex functions of absorption and transport of nutrients and drugs that we just saw, the intestine is the place where different unique features are combined: a considerable surface, achieved by intestinal villi and microvilli of the epithelial cells necessary for the absorption; an intestinal microbiota formed by billions of bacteria and peristaltic movements essential for the transport of nutrients along the gastrointestinal tract [2].
These characteristics are missing in the static models replicated in this gut-on-chip to properly mimic the human gut’s mechanical, structural, absorbent, and pathophysiological properties, combined with microbial symbionts. The main advantages of this advanced model are a better reproduction of the in-vivo environments, with controlled microfluidics parameters, and a reduction of the delays and costs of research.
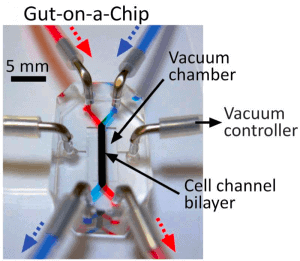
Technical characteristics of the gut-on-chip
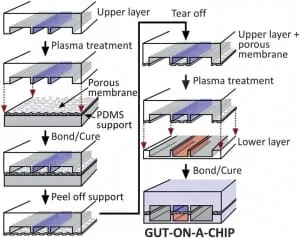
Hyun Jung Kim et al. [3] successfully designed a gut-on-chip with the previously listed characteristics of interest, so we decided to present their results in this review. The microdevice was composed of two aligned microchannels (upper and lower), made by casting polydimethylsiloxane (PDMS) prepolymer on a microfabricated mold and separated by a porous (10 um diameter circular pores) flexible membrane (also in PDMS prepolymer) coated with extracellular matrix (ECM) and lined by human intestinal epithelial cells that mimic the complex structures and physiology of living intestines.
They used the Caco-2BBE human colorectal carcinoma cell line for the human intestinal epithelial cells. This cell line, originally obtained from a human colon adenocarcinoma, has been extensively used over the last twenty years as a model of the intestinal barrier [4, 5].
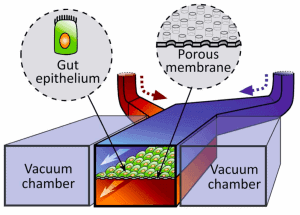
The physiological peristaltic motions and gut microenvironment were recreated by flowing fluid at a low rate (30 µl/h), producing low shear stress over the microchannels, and by exerting cyclic suction applied to the vacuum chambers positioned on either side of the microchannels, causing the porous membrane to stretch and relax periodically. A computer-controlled vacuum manifold regulated this cyclic suction.
The central role of the human microbiome in intestine function was also investigated: they performed a co-culture in the chip, using both Lactobacillus rhamnosus GG (LGG) cells, which were initially isolated from the human gut, and Caco-2 cells. These normal intestinal microbes were cultured on the apical surface of the epithelial cell monolayer.
Results obtained with the gut-on-chip
A parallel study using the Transwell chambers method was performed as control, to be able to correctly compare this static model with the gut-on-chip way of intestine cell culture. In these control studies, the Transwell plates contained porous polyester membrane inserts that were pre-coated with the same ECM mixture used in the gut-on-chip device; and the Caco-2 cells were plated at the same density to ensure the same basis of culture conditions.
At the end of both studies, they showed that cells grown under static conditions were highly flattened and almost squamous; whereas those in the gut-on-chip were 6-fold taller in size and developed a columnar shape that polarizes rapidly, spontaneously grows into folds that recapitulate the structure of intestinal villi.
Those characteristics were almost the same as reported for cells within healthy human intestinal epithelium in vivo, and shown to be directly dependent of the fluid flow exerted in the chip. This structure also forms a high integrity barrier to small molecules, which recapitulates another function of the intestinal cells. This integrity was established by measuring the transepithelial electrical resistance (TEER).
In addition, the culture of normal intestinal microbes for extended periods on the luminal surface of the cultured epithelium was a success. Indeed, the results showed that both LGG and Caco-2 cells remained fully viable after the co-culture. Microbes also brought significant advantages: they improved barrier function over time, as observed in humans, and increased intestinal epithelial integrity.
In the Transwell system, the LGG cells grew without constraint in the stagnant apical chamber, which caused acid Ph and low intestinal cell survival. This radical consequence was avoided in the gut-on-a-chip, thanks to the continuous flow exerted, causing the clearance of both organic acids and unbound LGG cells. The human intestinal epithelial cell functionality was measured by quantifying the specific activity of the aminopeptidase.
Further applications of the gut-on-chip device
This gut-on-chip provides a better model of the whole intestine than the previous Transwell chambers by recreating multiple dynamic physical and functional features of the human intestine critical for its function and a controlled microfluidic environment amenable for transport absorption and toxicity studies.
These recapitulations of the mechanically active microenvironment of the living intestine (peristaltic motions and intraluminal fluid flow) also permit an average growth of microbial flora without compromising human cell viability.
In summary, this device is a sound reproduction of the human intestine and thus has a promising future. The intestine is an essential organ that needs to be perfectly understood due to its complex combination of characteristics. This chip would facilitate the study of mechanoregulation of intestinal function and host-microbe symbiosis and evolution.
It also would offer a platform for drug screening and toxicology testing. Research on multiple gastrointestinal diseases, like Crohn’s disease, and their unknown causes would benefit from it. Animal models concerning tests on metabolism, transport, and oral absorption of drugs and nutrients could be avoided.
This current research method raises ethical controversies and provides results that are only sometimes applicable to humans, creating an urgency for developing alternatives. We could argue that this model is a significant simplification of reality, but it is possible to progressively add other features.
For instance, blood could be directly used as flowing fluid instead of the current medium. Another opportunity would be the development of a gut-liver-on-a-chip that could allow research on the first-pass effect in the metabolism of drugs and nutrients [6].
We recently published a review about the different organ-on-a-chip models and current innovations. If you are interested, have a look!
Authors:
Emilie Grandfils, Julie Cavallasca and Guilhem Velvé Casquillas
Contact:
Partnership[at]microfluidic.fr

References
- Caitriona M. Guinance and Paul D. Cotter, Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ, Therap Adv Gastroenterol. 2013; 6 (4): 295–308
- Hall JE. General principles of gastrointestinal function – motility, nervous control, and blood circulation. In: Hall JE, ed. Guyton and Hall Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Elsevier; 2016: chap 63
- Hyun Jung Kim, Dongeun Huh, Geraldine Hamilton and Donald E. Ingber, human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow, 2012; 12 (12):2165-74
- Hidalgo IJ; et al. (1989), Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability, Gastroenterology. 96 (3): 736–49
- Sambuy Y.; et al. (2005), The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics.
- Choe, A., Ha, S.K., Choi, I. et al. Biomed Microdevices (2017) Microfluidic Gut-liver chip for reproducing the first pass metabolism
- Written by Emilie Grandfils, corrected by Julie Cavallasca, under the supervision of Dr. Guilhem Velve Casquillas
Check the other Reviews
FAQ - Gut-on-chip: keeping up with the technology
What is a gut-on-chip and why do we need it?
A gut-on-chip is a microfluidic device that mimics the form and function of the intestine. It reconstructs important intestinal characteristics such as villi architecture, peristalsis, fluid flow, and host-microbe interaction. The significance of this technology is that the gut has important roles to play i.e., absorption of nutrients, drug metabolism, immunity via gut-associated lymphoid tissue, synthesis of vitamins and interaction with the brain through gut-brain axis. The study of these complex processes is essential for the development of treatments for gastrointestinal diseases and for testing the safety of drugs.
What are the issues that gut-on-chip devices solve over the traditional methods?
The use of traditional in vivo animal testing is ethically questionable and in most cases, leads to outcomes that cannot be generalized to humans since drugs could be harmful to animals but safe to humans or the other way around. Past in vitro systems, such as Transwell chambers, were simply a simple culture model having porous membranes, without key intestinal features of peristaltic motions, regulated fluid flow, and a high surface area of villi forms, and could not support intestinal microbiota. Gut-on-chip technology can help overcome these drawbacks by modeling the dynamic mechanical, structural, and pathophysiological characteristics of the living human intestine.
What are the most important technical peculiarities of a gut-on-chip device?
The device consists of two parallel microchannels filled with polydimethylsiloxane, with a porous, flexible membrane between and covered with extracellular matrix, with human intestinal epithelial cells (usually Caco-2BBE cells of human colorectal carcinoma) on the inside. The peristaltic motions are modeled using low-rate fluid flow (30 microliters per hour) with low shear stress, and cyclic vacuum chamber suction is used on the microchannels placed adjacent to the vacuum chambers. This makes the membrane stretch and relax every now and then, and it is controlled by a computer-regulated vacuum manifold that mimics natural intestinal motions.
What does the gut-on-chip do to recapitulate the intestinal microbiome?
The instrument manages to culture human intestinal epithelial cells in co-culture with normal intestinal microbes like Lactobacillus rhamnosus GG cells, which were first found in the human gut. These microbes are grown on the apical surface of the epithelial cell monolayer. The fluid flow in the chip is continuous, which is vital because it removes organic acids and free and bound bacteria cells by preventing the acidic pH and poor cell survival that would otherwise occur in stagnant Transwell systems, where bacteria grow freely in the chambers.
What are structural differences in cells in gut-on-chip compared to the case of static cultures?
The cells which are cultured on Transwell chambers under static conditions are really flattened and nearly squamous in form. Conversely, cells in the gut-on-chip are 6-fold taller, grow columnar, which forms a rapid polarization and forms folds spontaneously to recapitulates the structure of intestinal villi. These features are largely similar to those observed in cells in the normal intestinal epithelium in human beings in vivo and are directly dependent on the applied fluid flow in the chip. The structure also generates a high-integrity barrier to small molecules measured using transepithelial electrical resistance.
Why would it be advantageous to add microbes to gut-on-chip cultures?
There are great benefits of co-culture to the gut-on-chip system with normal intestinal microorganisms. Findings indicate that both the bacterial cells and the human epithelial cells do not lose their viability even after a long duration of co-culture. Microbes enhance the barrier and avert inflammatory processes over time like the case in living human intestines and enhance intestinal epithelial integrity. This is the ability to preserve microbial flora in a non-damaging way to the viability of human cells and therefore allows study of host-microbe symbiosis and interaction as microbial symbionts have a substantial impact on intestinal health and disease.
Mechanisms of gauging the functionality of intestinal cells in gut-on-chip systems?
The functionality of the intestinal epithelial cells is determined by the amount of a particular enzyme such as aminopeptidase which is an enzyme of significance in protein digestion and the absorption of nutrients. Barrier integrity can as well be determined through the transepithelial electrical resistance measurement that measures the extent to which the epithelial layer resists passage of molecules and ions. These assays prove that the gut-on-chip does not only replicate the structural characteristics but also functional properties of the living intestine, such as selective permeability and metabolic activity.
What are the potential gut-on-chip technology research applications?
Gut-on-chip is a platform that has been used in drug screening and toxicology testing, and also to study the mechanoregulation of intestinal function, host-microbe symbiosis and evolution, it has also been used to study the etiology of gastrointestinal diseases like Crohn’s disease, and in the study of metabolism, transport, and oral absorption of drugs and nutrients. The technology has the potential to minimize the use of animal models and to provide controlled microfluidic environments suitable for absorption and toxicity investigations.
What can be done with the future of gut-on-chip technology?
The model can be further developed by introducing new features. Instead of using culture medium, blood could be used as flowing fluid directly to model physiological conditions better. Studies of the first-pass effect in drug and nutrient metabolism, in which intestinal-absorbed substances are metabolised in the liver prior to entering systemic circulation, would be possible by the development of multi-organ systems like gut-liver-on-a-chip. The models that would be expanded would be used to understand increasingly complex processes in physiological systems and drug interactions.
Why is Caco-2 cell line a popular cell culture in gut-on-chip studies?
The human colorectal carcinoma cell line Caco-2BBE was first selected in 1980, and it has been widely used in the last two decades as an intestinal barrier model. These cells are well-defined and showcase significant events of the intestinal epithelial cells such as the capacity to form tight junctions, develop microvilli, express intestinal enzymes, and establish selective permeability boundaries. Their historical application in intestinal studies offers a supported basis of the gut-on-chip studies with considerable history to compare and interpret findings.

