Gut-on-chip: keeping up with the technology
Introduction to the gut-on-chip
Today's problematic
The gut fulfills many critical bodily functions, especially concerning the transport and oral absorption of drugs and nutrients, one of the essential phases of pharmacokinetics. There is also an immune function, which is less known and would require extensive research.
Despite this central role, we must understand all its mechanisms to develop an effective model of this complex and significant organ. Moreover, a lot of adverse drug effects affect the gastrointestinal tract, and a lot of gut inflammations have unknown causes, as is the case for Crohn’s disease.
Finally, there is growing evidence of the significant impact of microbial symbionts on the intestine’s health. This characteristic is not often investigated in vitro because of the difficulties in matching growth conditions between host cells and microbes.
Nevertheless, it remains a critical parameter because of the central role of the human microbiome in intestinal health and diseases [1], and the development of an in vitro platform to study host-microbe interplay is critical.
The Microfluidics Innovation Center developed a gut-on-chip pack.
Previous solutions
In vivo tests on this organ were mainly done on animals, which raises more controversy on the ethical front and provides results that cannot always be applied to humans: a drug can be toxic for rats and not for humans, and inversely.
A previous intestinal epithelial cell culture technique in vitro used mostly static culture models, as with the Transwell chambers (Corning Inc., Lowell, MA). This 3D cell culture stepped towards “in-vivo-like” environments with porous membranes and apical/basolateral sides for the chambers. Nevertheless, it is still missing crucial characteristics of the intestinal tissue to be a good predictive model of the intestine.
The gut-on-chip solution
Given the complex functions of absorption and transport of nutrients and drugs that we just saw, the intestine is the place where different unique features are combined: a considerable surface, achieved by intestinal villi and microvilli of the epithelial cells necessary for the absorption; an intestinal microbiota formed by billions of bacteria and peristaltic movements essential for the transport of nutrients along the gastrointestinal tract [2].
These characteristics are missing in the static models replicated in this gut-on-chip to properly mimic the human gut’s mechanical, structural, absorbent, and pathophysiological properties, combined with microbial symbionts. The main advantages of this advanced model are a better reproduction of the in-vivo environments, with controlled microfluidics parameters, and a reduction of the delays and costs of research.
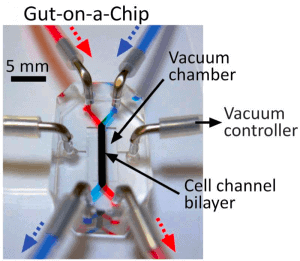
Technical characteristics of the gut-on-chip
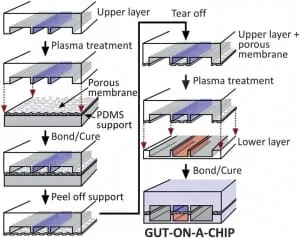
Hyun Jung Kim et al. [3] successfully designed a gut-on-chip with the previously listed characteristics of interest, so we decided to present their results in this review. The microdevice was composed of two aligned microchannels (upper and lower), made by casting polydimethylsiloxane (PDMS) prepolymer on a microfabricated mold and separated by a porous (10 um diameter circular pores) flexible membrane (also in PDMS prepolymer) coated with extracellular matrix (ECM) and lined by human intestinal epithelial cells that mimic the complex structures and physiology of living intestines.
They used the Caco-2BBE human colorectal carcinoma cell line for the human intestinal epithelial cells. This cell line, originally obtained from a human colon adenocarcinoma, has been extensively used over the last twenty years as a model of the intestinal barrier [4, 5].
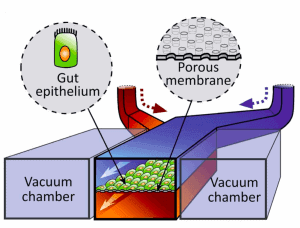
The physiological peristaltic motions and gut microenvironment were recreated by flowing fluid at a low rate (30 µl/h), producing low shear stress over the microchannels, and by exerting cyclic suction applied to the vacuum chambers positioned on either side of the microchannels, causing the porous membrane to stretch and relax periodically. A computer-controlled vacuum manifold regulated this cyclic suction.
The central role of the human microbiome in intestine function was also investigated: they performed a co-culture in the chip, using both Lactobacillus rhamnosus GG (LGG) cells, which were initially isolated from the human gut, and Caco-2 cells. These normal intestinal microbes were cultured on the apical surface of the epithelial cell monolayer.
Results obtained with the gut-on-chip
A parallel study using the Transwell chambers method was performed as control, to be able to correctly compare this static model with the gut-on-chip way of intestine cell culture. In these control studies, the Transwell plates contained porous polyester membrane inserts that were pre-coated with the same ECM mixture used in the gut-on-chip device; and the Caco-2 cells were plated at the same density to ensure the same basis of culture conditions.
At the end of both studies, they showed that cells grown under static conditions were highly flattened and almost squamous; whereas those in the gut-on-chip were 6-fold taller in size and developed a columnar shape that polarizes rapidly, spontaneously grows into folds that recapitulate the structure of intestinal villi.
Those characteristics were almost the same as reported for cells within healthy human intestinal epithelium in vivo, and shown to be directly dependent of the fluid flow exerted in the chip. This structure also forms a high integrity barrier to small molecules, which recapitulates another function of the intestinal cells. This integrity was established by measuring the transepithelial electrical resistance (TEER).
In addition, the culture of normal intestinal microbes for extended periods on the luminal surface of the cultured epithelium was a success. Indeed, the results showed that both LGG and Caco-2 cells remained fully viable after the co-culture. Microbes also brought significant advantages: they improved barrier function over time, as observed in humans, and increased intestinal epithelial integrity.
In the Transwell system, the LGG cells grew without constraint in the stagnant apical chamber, which caused acid Ph and low intestinal cell survival. This radical consequence was avoided in the gut-on-a-chip, thanks to the continuous flow exerted, causing the clearance of both organic acids and unbound LGG cells. The human intestinal epithelial cell functionality was measured by quantifying the specific activity of the aminopeptidase.
Further applications of the gut-on-chip device
This gut-on-chip provides a better model of the whole intestine than the previous Transwell chambers by recreating multiple dynamic physical and functional features of the human intestine critical for its function and a controlled microfluidic environment amenable for transport absorption and toxicity studies.
These recapitulations of the mechanically active microenvironment of the living intestine (peristaltic motions and intraluminal fluid flow) also permit an average growth of microbial flora without compromising human cell viability.
In summary, this device is a sound reproduction of the human intestine and thus has a promising future. The intestine is an essential organ that needs to be perfectly understood due to its complex combination of characteristics. This chip would facilitate the study of mechanoregulation of intestinal function and host-microbe symbiosis and evolution.
It also would offer a platform for drug screening and toxicology testing. Research on multiple gastrointestinal diseases, like Crohn’s disease, the unknown causes, would benefit from it. The animal models concerning tests on metabolism, transport, and oral absorption of drugs and nutrients could be avoided.
This current research method raises ethical controversies and provides results that are only sometimes applicable to humans, creating an urgency for developing alternatives. We could argue that this model is a significant simplification of reality, but there is a possibility of progressively adding other features.
For instance, blood could be directly used as flowing fluid instead of the current medium. Another opportunity would be the development of a gut-liver-on-a-chip that could allow research on the first-pass effect in the metabolism of drugs and nutrients [6].
Authors:
Emilie Grandfils, Julie Cavallasca and Guilhem Velvé Casquillas
Contact:

References
- Caitriona M. Guinance and Paul D. Cotter, Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ, Therap Adv Gastroenterol. 2013; 6 (4): 295–308
- Hall JE. General principles of gastrointestinal function – motility, nervous control, and blood circulation. In: Hall JE, ed. Guyton and Hall Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Elsevier; 2016: chap 63
- Hyun Jung Kim, Dongeun Huh, Geraldine Hamilton and Donald E. Ingber, human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow, 2012; 12 (12):2165-74
- Hidalgo IJ; et al. (1989), Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability, Gastroenterology. 96 (3): 736–49
- Sambuy Y.; et al. (2005), The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics.
- Choe, A., Ha, S.K., Choi, I. et al. Biomed Microdevices (2017) Microfluidic Gut-liver chip for reproducing the first pass metabolism
- Written by Emilie Grandfils, corrected by Julie Cavallasca, under the supervision of Dr. Guilhem Velve Casquillas