C. elegans immobilization via microfluidics: a review
Author
Walter Minnella, PhD
Publication Date
May 16, 2015
Keywords
temperature-induced immobilization
chip-gel hybrid
Carbon dioxide immobilization
Microfluidic clamps array

Need advice for your C. elegans immobilization?
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Introduction to C. elegans immobilization
Caenorhabditis elegans, commonly shortened as C. elegans, is a non-parasitic nematode widely used as a model organism in biology for its peculiar proprieties. Indeed, C. elegans, since Sydney Brenner’s pioneering studies in the 1970s [1], has been the subject of several investigations, primarily focused on its nervous system as it is one of the simplest organisms having one.
This interest led to the C. elegans being the first multicellular organism to have its whole genome sequenced. The vast knowledge and its unique transparency features make C. elegans a choice of election for biologists in live cell imaging.
The most essential prerequisite to achieving high-resolution, live cell videos and images of C. elegans is the immobilization of the animal. Indeed, contingent movements can raise artifacts or out-of-focus images, making the whole observation useless. Immobilization methods can be divided into two general approaches: chemical techniques and physical techniques.
C. elegans immobilization usig microfluidics
The first category embraces all methods that achieve immobilization by any chemical compounds, such as metal ions or antibodies. Although these techniques can provide good results in terms of immobilization, most of them appear to affect the cells adversely, even using deficient concentrations.
The physical methods, on the other hand, generally depend on alterations of viscosity of the medium through or on some means of capturing and holding the worm. A more detailed and exhaustive review of these methods can be found in Aufderheide et al. 2008 [2].
Concerning C. elegans, the most common techniques found in the literature to obtain the worms’ immobilization rely on glue or anesthetic drugs. Although these methods are well-established, they can potentially affect the sample, inducing physiological modifications or preventing future manipulation. Furthermore, they are low-throughput techniques as it is rather tricky and time-consuming to immobilize many nematodes with these methods.
Even using a more recent and sophisticated means to obtain C elegans immobilization, such as polystyrene nanoparticles [3], while it is easier to set up, has drawbacks. The most restrictive one is that immobilization, in this case, depends on worm growth stages and thus requires a presorting of the worm’s population.
Microfluidics can be the answer to this and other issues regarding the study of C. elegans, as it not only allows controlled flows and drug delivery to the sample but also provides a platform for easy animal manipulation and handling, which is otherwise manual and time-consuming. Additionally, the use of glues and anesthetics for studies that require worm immobilization is avoided.
This review’s objective is to describe the most common microfluidic device and techniques to achieve C. elegans immobilization.
Recently, many methods and devices in microfluidics have been developed to obtain on-chip worm immobilization. The most notable have achieved immobilization by cooling [4], compression or restriction [5, 6], CO2 [5], and gelation of the surrounding fluid [7]. In the following section, these techniques will be briefly presented.
Microfluidic temperature-induced immobilization of C. elegans
This method, developed by Chung et al. [4], addresses the critical challenge of in vivo microscopy, phenotyping, and screening of having hardware capable of handling worms in a way compatible with standard readouts and that allows automation of observation and manipulation of the sample.
This technique employs a polydimethylsiloxane (PDMS) microfluidic chip with built-in valves to control a suspension of nematodes. This device’s crucial feature lies in integrating several key design features that ensure experiments with high throughput. Additionally, an algorithm can be employed to automate the entire image acquisition, analysis, and sorting process, allowing the system to operate without human intervention.
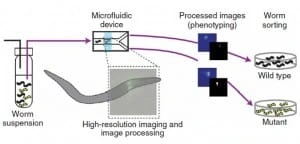
Chung’s protocol relies on four steps (Figure 1): worms are first loaded into the microfluidic device by a constant presure-driven flow, then the sample is immobilized reversibly by cooling and thus scanned by a high-resolution microscope. Subsequently, phenotyping and sorting occur, and the obtained videos/images are stored for further analysis.
Conventional soft lithography methods manufacture the microfluidic chip. It comprises two layers, one for flow regulation and one for temperature control, divided by a 20 µm thick PDMS membrane (Figure 2). The layers are bonded to the cover glass via oxygen plasma treatment. Six salient features characterize the microfluidic device:
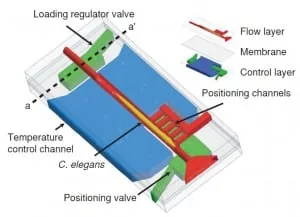
- A built-in system automatically regulates the loading of nematodes inside the chip, ensuring that just one worm at a time is inside the imaging chamber.
- A high-precision positioning system automatically places each animal in the exact location.
- A temperature control system provides the cooling needed to immobilize the sample.
- The setup is fully compatible with any standard microscopy equipment.
- The setup is low-cost and relatively easy to manufacture.
- The setup provides high throughput and efficient sorting.
To load a worm into the chip, both outlet channels are closed while the side positioning channels remain open. The flow is then driven into channels by a constant pressure source. Furthermore, a system of loading regulators ensures that only one worm at a time is present in the area of the chip designated for imaging.
This is possible because when an animal is present in the imaging chamber, the flow resistance of the chamber increases as a result. The flow rate – and hence the pressure – lowers on a second animal at the sample-loading regulator located at the imaging chamber’s entrance.
Consequently, this pressure drop makes it impossible for the system to push the second worm inside. Then, as the first worm is released, this constraint is lifted, and the pressure rises to a point where the system can push inside the next worm. To achieve precise and reproducible positioning of the warmth inside the imaging chamber, the pressure difference between the positioning channels and the entrance of the main channel is exploited.
Indeed, once the animal is positioned inside the detecting zone, the hydrodynamic resistance of the positioning channels self-equalizes, and thereby, the pressure is equally distributed on the worm’s body, preventing too high mechanical stress on the animal. This high-precision placing feature of the system minimizes the travel of the motorized stage of the microscope to locate the worm and makes the whole process less time-consuming.
Once placed in the imaging area, nematodes are cooled to ~ four °C to immobilize them. This procedure does not employ any anesthetic and thus does not have any potential side effects on the sample due to drug use. Furthermore, it has been proven to be as effective as immobilization by sodium azide. Finally, using microfluidic allows this setup to be easy and cheap to replicate and fully compatible with any standard microscopy setup.
Carbon dioxide immobilization of C. elegans on microfluidic chips
Chokshi et al. [5] developed a technique for C. elegans immobilization that takes advantage of a microfluidic chip with a built-in immobilization chamber where the worm is trapped by creating a CO2 micro-environment. Indeed, carbon dioxide can inhibit the nematode’s movement for extended periods (up to 2 hours) without causing permanent damage to the worm, which can recover a few seconds after immobilization. Moreover, this method has been proven suitable for worms of different age groups (L4s to adults).
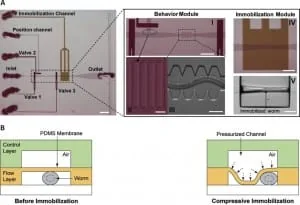
The device employed is a two-layer PDMS microfluidic chip (Figure 3): one layer, called the “behavior” module, is deputed to the revitalization of the animal after immobilization, while the other one is where the worm gets immobilized and thus is named immobilization module.
The behavior module consists of a saw-shaped microchannel that forces the worm to move in a sinusoidal pattern so that its locomotion can be further analyzed. The immobilization layer contains the chamber where the carbon dioxide micro-environment is created to immobilize the worm to perform imaging. Finally, the two layers are separated by a 30 µm-thick PDMS membrane.
The CO2 micro-environment is obtained by letting pure carbon dioxide flow through the control layer, which diffuses through the membrane into the flow layer. As well known, PDMS is highly permeable to non-polar gases. Hence, carbon dioxide can quickly replace the air in the immobilization chamber. Moreover, the membrane is thick enough to sustain the gas pressures (10 psi) without noticeable deflections.
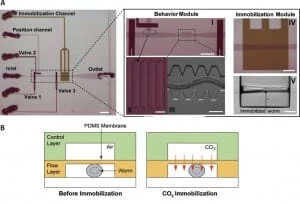
Worms are loaded into the main flow channel of the chip and manipulated by activating the integrated microfluidic valves (valves 1, 2, and 3 in Figure 3) via the control channel. A separate channel (the “position” channel) perpendicular to the central loading channel positions the worm inside the behavior and immobilization modules. Some PDMS pillars (Figure 3, II) are fabricated at its intersection with the loading channel to prevent the worm from entering the position channel.
This technique can achieve extended periods of immobilization (up to 2 hours), thus allowing more extended periods for microscope observation. Moreover, the lack of oxygen in the immobilization chamber reduces the photobleaching of fluorescent markers, making the CO2 method particularly appealing for long-term fluorescence imaging experiments such as time-lapse imaging of cell development.
Microfluidic array of clamps for C. elegans trapping
Hulme et al. [6] designed a microfluidic device capable of mechanically trapping large (>100) numbers of C. elegans at once. The procedure relies on implementing a microfluidic chip of wedge-shaped channels (Figure 4), or “clamps”, which can trap the worm and thereby physically inhibit its movements. In particular, the width of the microchannel narrows gradually, lowering from 100 µm to 10 µm over a length of 5 mm (Figure 4).
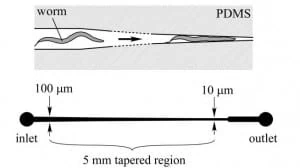
The device consists of a network of distribution channels that supply an array of these clamps via a constant-pressure flow that drives the animals. As the immobilization takes place without drug treatment or any other potentially harmful measures, this method does not disturb the natural biochemical state of the worm. Moreover, worms are released from the traps by reversing the flow direction, and repeated sampling of the same population is therefore possible.
A constant pressure difference between the chip’s inlet and outlet is required to drive and trap the worms into the clamps. It is essential to underline that constant pressure and not a constant flow is needed in this case, as the unbounded increase in pressure resulting from a continuous flow regime could cause significant mechanical damage to the immobilized worms.
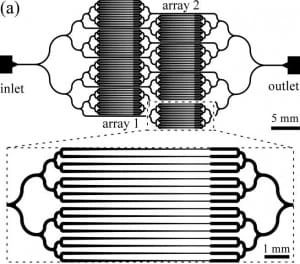
Figure 5 shows a schematic of the microfluidic device, which is made of an array of worm clamps. Channels are designed to tape gradually so worms of different sizes can be accommodated as, even in an isogenetic population, noticeable size differences between the individuals can be found. Therefore, the system can also be employed as an analytic device to measure the size distribution of a given population of worms.
Moreover, bifurcation over higher-order branching topologies has been chosen to avoid bias in worms’ distribution inside the chip. Finally, as a trapped worm increases the fluidic resistance of a given channel, it will not follow the same path when another worm arrives at the same junction; thus, the device is designed to load an offered clamp with only one worm. It is essential to notice, though, that no control is provided over the orientation of the worm when immobilized, i.e. if it gets trapped head-first or tail-first.
To wrap up, this technique allows, in quite a short period (~15 min), to reversibly immobilize many nematodes without any chemical compound. This can help the researcher save time during their experiment and ensure a high throughput during all observation time.
Moreover, using a microfluidic device leaves the possibility of further improvements in the number of nematodes that can be trapped and of implementing new features by adding other microfluidic components, e.g., for drug injection. Finally, as the immobilization device creates an ordered array of trapped worms, it should be possible, in principle, to automate the acquisition of data using a motorized microscope stage.
Compressive immobilization of C. elegans on microfluidic chip
This technique has been developed by Chokshi et al. [5], and it is a variation of the carbon dioxide method described before (cf. §3). Indeed, the device design is almost identical – a two-layer PDMS chip – with the only difference lying on the membrane that divides the two layers. Certainly, this time, the animal immobilization is achieved mechanically: a high-pressure (25 psi) airflow causes the membrane to deflect on the worm, compressing it and, as a result, inhibiting its movements (Figure 6).
A few changes are made to make the membrane more deformable to maximize the immobilization effect. First, a 20:1 PDMS/curing agent mixing ratio is used rather than the conventional 10:1; this decreases the PDMS elastic modulus by two. Moreover, the width of the microchannel in the immobilization module is made wider (110 µm) than the width of the saw-shape micro- channel (90 µm) to allow large membrane deflections.
The advantage of this method lies in the ease of manufacturing the microfluidic device and its further implementation. Moreover, this technique is suitable for animals of different ages and can be applied serially to increase the throughput of giant worms. Finally, a shortcoming of this method is the relatively short (minutes) immobilization time achievable compared to other techniques.
C. elegans immobilization in chip-gel hybrid microfluidic device
The methods described before are very efficient for morphological and phenotype investigation; nevertheless, they present some shortcomings. Indeed, either they do not allow longitudinal studies of physiologically active processes (e.g., cooling method, see §1) or induce shifts in the anatomical features of the worm due to the compression employed to immobilize them (e.g., clamps and compressive method, cf. §3 and §4).
Krajniak and Lu [7] addressed these issues by designing a hybrid microfluidic chip-gel system that allows the performance of long-term high-resolution imaging of specific development processes at physiological conditions. In particular, this device can culture, immobilize, and image the nematodes all at once.
Reversible immobilization of C. elegansis was achieved by using a commercially available bio-compatible polymer: Pluronic F127 (PF127). This polymer is capable of reversible sol-gel transition, which a slight temperature shift can thermally induce (~2 °C). In particular, when the PF127 is in its gel state (gelification), its viscosity increases at a level that inhibits animal movements. The microfluidic device is a PDMS-based two-layer chip (Figure 7), where a thin PDMS membrane separates the two layers.
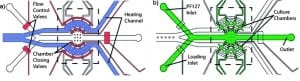
One module is deputed for load and flow control, whereas the other is exploited for temperature control. Both channels have a thickness of 3 mm, while the membrane between them is 30 µm. The chip’s two layers are bonded with a conventional oxygen plasma treatment. A vital feature of this device is that worms are cultured within the device, as the latter can provide nutrient and gas exchange to nematodes inside its eight chambers (Figure 8).
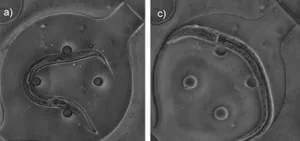
Furthermore, these chambers keep animals separated during all investigation time, ensuring that development can be followed on a single-animal basis. The flow layer contains all flow inlets used to load the worms and supply the chamber with the PF127 solution and the individual culture chambers.
Moreover, pneumatic valves are used to prevent the escape of nematodes from the culture chambers. The second layer, the temperature control module, produces small (a few °C) temperature changes on the device to trigger the PF127 transition.
Precise temperature control is achieved by regulating the flow rate of the heating fluid and maintaining the heating fluid source temperature. As said previously, the immobilization mechanism relies on a solution of PF127, which leads into the culture chamber and goes under a reversible sol-gel transition triggered by thermal shifts. Thus, immobilization occurs anywhere in the chamber without dependence on the worm placement and minimal environmental changes.
In conclusion, this method can immobilize up to 8 worms at a time for a long time without interfering with their biological functions. Furthermore, the immobilization protocol is fully reversible for an undefined number of times.
Conclusion
This review presents some of the most notable microfluidic methods to achieve C. elegans immobilization. In particular, the apparent advantages of exploiting microfluidics for this goal have been underlined. Indeed, conventional immobilization methods, which rely mainly on glue or anesthetic drugs, have been proven to be time-consuming, have low throughput, and are potentially hurtful for the sample compared to microfluidic alternatives. The obvious question arising now is: which microfluidic technique should I choose?
There is no definite answer to this question, as which technique is the best for you strongly depends on your requirements and needs. Moreover, each of these methods ensures excellent results in terms of C. elegans immobilization. Among them, Chokshi’s approach provides the best compromise regarding manufacturing difficulties over performance.
The microfluidic device employed is, in fact, relatively simple to realize as it is a two-layer PDMS chip with a simple array of channels. In addition, the same design can be used for two immobilization techniques (compressive and CO2) if the researcher requires either short (minutes) or long (hours) immobilization periods. Furthermore, this method can be applied to worms of different age groups and fully recover the animal from immobilization.
Review written with the support of the LAPASO project
FP7-PEOPLE-2013-ITN–Marie-Curie Action:
“Innovative Training Networks”
Author: Walter Minnella, PhD
Contact:
Partnership[at]microfluidic.fr


References
- Brenner, S. (1974). The Genetics of Caenorhabditis elegans. Genetics 77 (1): 71–94.
- Aufderheide, K. J. (2008). An overview of techniques for immobilizing and viewing living cells. Micron 39, 71–76
- Kim E., Sun L., Gabel C. V., Fang-Yeng C. (2013). Long-term imaging of Caenorhabditis elegans using nanoparticle-mediated immobilization. PLoS ONE 8(1): e53419
- Chung K., Crane M. M., and Lu H. (2008). Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637-643.
- Chokshi T. V., Ben-Yakar A., and Chronis N. (2009). CO2 and compressive immobilization of C. elegans on-chip. Lab Chip 9, 151-157.
- Hulme S.E., Shevkoplyas S.S., Apfeld, J., Fontana W., and Whitesides G.M. (2007). A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab Chip7, 1515-1523.
- Krajniak, J., & Lu, H. (2010). Long-term high-resolution imaging and culture of C. elegans in chip-gel hybrid microfluidic device for developmental studies. Lab on a Chip, 10(14), 1862-1868.
As well as articles cited in these references.
Check the other Reviews
FAQ - C. elegans immobilization via microfluidics: a review
What is C. elegans and why is it significant to biological research?
Caenorhabditis elegans is a non-parasitic nematode or roundworm commonly used as a biology model organism, having been first studied in the 1970s by Sydney Brenner. It became the first multicellular organism with the complete genome sequence. C. elegans is preferred by researchers due to its simple and functional nervous system, notable transparency allowing in vivo imaging of cells, short lifespan, simple culture, and well-developed genetics. These characteristics render it perfect when it comes to studying development, neurobiology, aging and disease mechanisms.
What is the rationale of immobilizing C. elegans?
High-resolution microscopy and live cell imaging of C. elegans demands that the animal remains fully stationary since any movement will produce artifacts or out-of-focus images that will render the observational process useless. Conventional imaging methods cannot cope with the undesirable incessant natural motions of the nematode. The first requirement is immobilization which is the necessary pre-requisite to acquiring clear and high quality videos and images required to perform detailed cellular and sub cellular analysis, phenotyping and long term developmental studies.
What is the mechanism of temperature induced microfluidic immobilization?
The cooling mode involves reversibly immobilizing worms with a PDMS microfluidic chip with an inbuilt temperature control to cool them down to about 4degC. The two-layer device is automated in loading, so that one worm is loaded in the imaging chamber at a time, there is high-precision positioning that ensures each animal is in the precise location and temperature regulation which is achieved by controlled fluid flow. Cooling immobilization is effective as deconjugation with chemical anesthetics without side effects of drugs, full recovery, and it can be used with the normal microscopic hardware but provides high-throughput automated screening.
What are the benefits to carbon dioxide immobilization?
CO2 immobilization introduces a carbon dioxide micro-environment within a closed chamber through flow of pure CO2 via a control layer diffusing through a PDMS membrane to the immobilization chamber. This approach is able to cause long term stagnation of motion to the 2 hour limit without permanent harm, and the worms resume their activities in a few seconds following paralysis. The method is effective regardless of the age of L4 larvae up to adults. More importantly, through oxygen-depleted environment, photobleaching of fluorescent markers is minimized and is therefore especially relevant in long-term fluorescence imaging as well as time-lapse research of cellular development.
What are the trapping mechanisms of the C. elegans by microfluidic clamp arrays?
The clamp array technique involves the microchannels in the shapes of wedges that become narrower towards the end of a 5-millimeter length by 100 micrometers to 10 micrometers. These channels are forced open by the continuous flow of pressure, the worms are forced into these channels and get trapped in the channels, which are mechanically constrained as the width of the channels is reduced. The device is bifurcating with the distribution channels designed to provide an even distribution of the worms with the design being such that only one worm can fit in the clamp because the channels become more resistant to fluid entering when occupied. It is possible to achieve paralysis of more than 100 nematodes at once in just 15 minutes with no chemical treatment, which has reversible trapping of the population through flow reversal and re-sampling of the identical population.
What is compressive immobilization and its difference to other methods?
Compressive immobilization involves subjecting the worm to the high pressure air flow (25 psi) to force a thin film of PDMS on to it physically compressing and immobilizing it. A 20: 1 ratio of PDMS to curing agent is used to make the membrane deformable rather than the usual 10:1 and this reduces the elastic modulus by half. Immobilization chamber is broader than the other channels to ensure that there is more membrane deflection. Although this technique is easy to use and applicable with various ages of worms, it offers comparatively very short durations of immobilization of just a few minutes in comparison to the hours of immobilization of the worms through other methods.
Why is the chip-gel hybrid system a way of facilitating the long-term studies?
The chip-gel hybrid system employs the biocompatible polymer, Pluronic F127, which sol-gel transition can change reversibly in small temperature variations of about 2degC. In the gelified state, the viscosity is high and in this condition worms cannot be compressed or cooled as may influence physiology. This device has eight individual culture chambers, which enable long term culturing, nutrient exchange, gaseous exchange and tracking of development in single animal. This system makes possible the physiological relevance of conditions in immobilization, maintenance of anatomical structures devoid of compression artifacts, and the repeated immobilization cycles to conduct longitudinal studies of active biological processes.
What microfluidic immobilization method are researchers supposed to follow?
They are chosen on certain experimental conditions. Temperature-induced immobilization is very automatable to short-term high-throughput phenotyping and automated sorting. CO2 immobilization is preferred in long-term fluorescence imaging to reduce the effects of photobleaching. Clamp arrays are efficient in fast parallel imaging of large numbers of worms and in the absence of chemicals. The chip-gel hybrid is the best option in longitudinal developmental studies that need physiological conditions. The use of a CO2 approach or compression approach by Chokshi provides reasonable versatility with comparatively easy fabrication of the equipment, and gives short or long periods of immobilization using the simple basic design.
What are the main strengths of microfluidic immobilization compared to the traditional ones?
Microfluidic systems have several features such as high throughputs with the capability to test many animals at once or in a sequence, automation capability to screen and phenotype, reversible immobilization that allows recovery of the specimen and reuse, elimination of potentially toxic chemical anesthetics, precise environmental control of temperature and gas mixtures, simultaneous functionality of many functions such as loading, positioning, immobilization and imaging, and compatibility with standard microscopy methods. These advantages allow microfluidic immobilization to be quicker, more consistent, and less harmful of specimens than conventional glue or drug-mediated processes.

