IgGs screening for neurodegenerative diseases diagnosis: AUTOIGG
Author
Christa Ivanova, PhD
Publication Date
September 14, 2021
Status
Keywords
neurodegenerative diseases
Auto-antibodies
amyotrophic lateral sclerosis
personalized medicine
neurons, glial cells
prognostic biomarkers
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Perfusion system for IgGs screening: introduction
The project aims to produce an innovative automated device for diagnosing neurodegenerative diseases.
The consortium comprises partners from Serbia (as coordinator), Finland, France, Turkey, and overseas partners, two from the USA and one from Costa Rica.

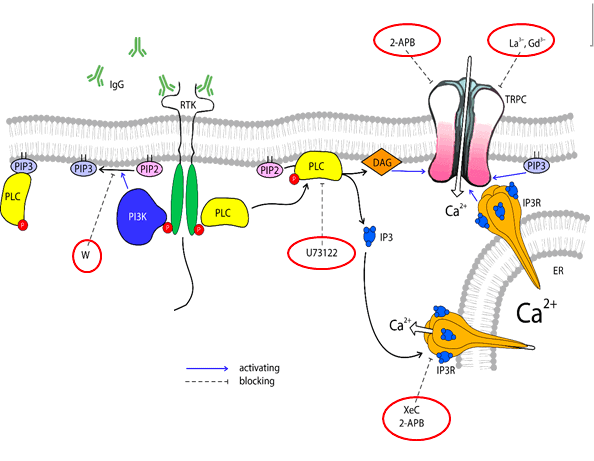
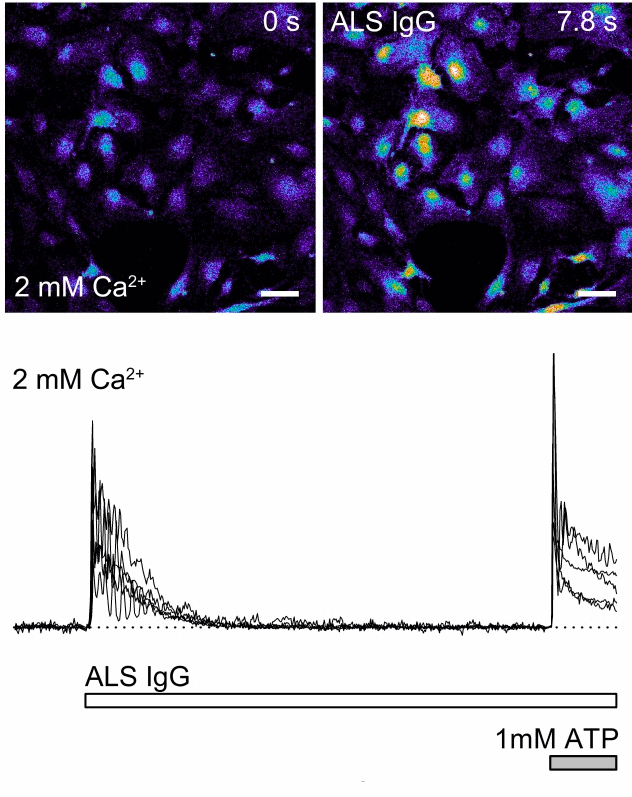
Immunoglobulins (IgGs) are the key players in immune responses neutralizing pathogens. In the AUTOIGG project, we recruit IgGs derived from patients suffering from neurodegenerative diseases, particularly amyotrophic lateral sclerosis, to study their effect on neurons and glial cells.
In the light of developing the automated device, one of our goals is a small-scale perfusion system with microchambers on a chip suitable for fluorescent live imaging in individual cells or cell groups.
Perfusion system for IgGs screening: our role
Live imaging is a powerful technique that allows the study of the effects of various compounds in cell culture, quantifying responses of individual cells. Perfusion chambers and fast application systems, which are commonly used for live imaging, have many advantages. However, we aim to improve the consistency of experiments with the help of microfluidics.
The first problem to solve is the shear stress induced by the everyday work of the application system. This shear stress activates mechanosensitive receptors, thus causing unwanted “artificial” responses that can overwhelm signals from compounds of interest. These artifacts become especially detrimental when the studying compound, such as IgGs, induces comparably small responses that must be carefully analyzed.
Secondly, existing perfusion chambers have rather significant volume and require an unacceptably large amount of studying solution, while in the case of IgGs derived from patients, sample volumes are minimal.
Thirdly, the design features of the existing macro-scale perfusion chambers and application systems require readjustments before each experiment, which leads to variation in spatial configuration and, hence, differences in final concentration and delivery time of applying the solution.
All three problems can be easily solved by combination of highly precise chips with embedded perfusion channels and our perfusion system.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 778405 (AUTOIGG).



Check our Projects
FAQ – IgGs screening for neurodegenerative diseases diagnosis: AUTOIGG
What are the problems that AutoIgG attempts to solve?
Neurodegenerative diseases are insidious, and over a period of time, classic serology usually records only blunt late-stage indicators. AutoIgG is the bridge gap: a high-speed microfluidics platform that effectively screens patient IgG repertoires, how antibodies actually bind, and perturb disease-relevant targets, providing clinicians and researchers with early, higher-resolution insights than a typical ELISA.
What is the difference between a classic ELISA or titer test?
Two things. First, the readout can actually work. But also, does it regulate the target, the pathway, or a cellular proxy? Second, the system can process a wide range of epitopes or conformers concurrently under strictly defined microflows, allowing you to interrogate specificity, affinity trends, and cross-reactivity in a single run rather than introducing multiple rounds over a week.
Why use microfluidics?
Deterministic transportation and small volumes. That implies stronger association/dissociation kinetics, fewer mass-transport artifacts, and much less reagent. In practice: an increased signal-to-noise, reduced assays, and a minimum level of precision to distinguish minor differences between polyclonal human IgGs that would be obscured by bulk.
Which biological targets are covered by neurodegeneration?
Confirmation-sensitive proteins and aggregates, Ab variants, tau (phosphorylated and truncated), and monomers/oligomers of α-synuclein, TDP-43 fragments, and peptides linked to the disease. The panel can combine both native-like and engineered architectures to obtain clinically relevant binding patterns without biasing toward any single epitope.
What is the actual measurement of functional screening?
Several layers: (i) binding to different conformers, (ii) competition or inhibition by reference antibodies, (iii) response to reporter display systems (e.g., seeded aggregation or cellular viability/readouts in a micro-co-culture companion chip). The mixture eliminates unspecific stickiness, as opposed to non-specific mechanism-linked antibody activity.
What is the multiplexing of the assay with no crosstalk?
The samples are directed via the chip to isolated channels or droplet lanes, each with its own immobilized target or bead code. Carryover is prevented by valving and timed staggering; the timing of the washing steps is under pressure, not reliant on a pipette. Barcoding is an optical or bead-ID method for ensuring identities are maintained throughout the run.
Throughput and sample economy, what do you, lab, think?
Common screening modes can deal with cohort-type batches (dozens of sera per day) containing less than 10 µL of starting material per condition. Since the platform can be expanded by increasing the number of channels, but not the plate size, adding lanes results in almost linear increases in throughput with little change in kinetics or wash quality.
Analytical performance: What is the way that reproducibility is maintained?
Constant pressure flow, on-chip reference channels, and per-run calibration curves. All panels are anchored with internal controls (positive/negative IgGs, isotype controls), and coefficients of variation are kept low through the design (tight residence-time control, identical shear histories). Practically, an identical panel of samples can be recalculated, and the same rank order is obtained, which is vital in longitudinal studies.
What was the contribution of Microfluidics Innovation Center (MIC)?
The design and microfabrication of a device, automation of the assay (timing, wash and incubation logic), and transition to a prototypical device that has standard operating procedures. Due to the sensitivity of fragile conformers, lower non-specific binding on surfaces, and the packaging of the workflow, MIC tuned flow regimes so that clinical or pharma partners can run the workflow and not be forced to babysit the chip over hours.
What is the place of AutoIgG in a diagnostic pipeline?
Prior to imaging and CSF-intensive operations. It is best used in the early stages of stratification to select candidates for more invasive testing and to follow up immunological signatures after therapy. It is useful in R&D to rank discovery antibodies and map epitope landscapes and make a decision to invest in animal or trial-grade studies.
Does the panel represent a customized panel for a specific study or rare phenotype?
Yes. Targets, conformer chemistries, buffer chemistries, and temperature regimes can be adjusted. You can bias the panel, e.g., toward phosphorylated tau isoforms or α-synuclein oligomers, and MIC can reconfigure surface chemistries and channel layouts for those purposes without reprinting the entire instrument.
What is the rationale of incorporating a microfluidics SME such as MIC in a Horizon consortium?
Due to the difference between a beautiful figure and a test that can be deployed, an engineering discipline is required. MIC is an expert in assay automation, fluidic reliability, and tech transfer, regularly collaborating on co-writing European proposals and prototyping to enable rapid availability to users. Consortia with an integrated microfluidics SME like MIC have been found in our experience to outperform formal proposal success baselines, usually about twice the chances, due to believable implementation, exploitation strategies, and validation paths.