Stem Cell Culture Platform
Automated and fail-safe long-term microfluidic cell culture system
Highly controlled microenvironment
Continuous and controlled supply of induction factors in a stable flow
Up to 3-week long cell cultures
Ideal for stem cell differentiation
Fail-safe mechanism
No more losing your cell experiment due to clogging
Automated sequences
Personalize sequences with our software for a tailor-made protocol

Need a microfluidic SME partner for your Horizon Europe project?
Human stem cell embedded in a 3D matrix, Cryo SEM. Ferreira, Silvia A. 2015. Licence: Attribution 4.0 International (CC BY 4.0)
Stem cell culture
Human-induced Pluripotent Stem Cells (hiPSC) carry the genetic information of their donor, having the potential to become great models for drug screening of disease phenotypes [1, 2]. In this light, hiPSC can be maintained in self-renewal mode, where the cells keep growing and dividing without differentiating and losing pluripotency. Alternatively, they can be incited to differentiate into the desired phenotype.
These processes are governed by cell culture conditions, especially the biomarkers present in the media [1]. Once triggered, the hiSPC differentiation can take a few days [3, 4] to weeks [5]. Microfluidics has been shown to improve the outcomes of stem cell culture. However, culturing cells for long periods inside microfluidic systems can be challenging.
Challenges of long-term microfluidic cell culture
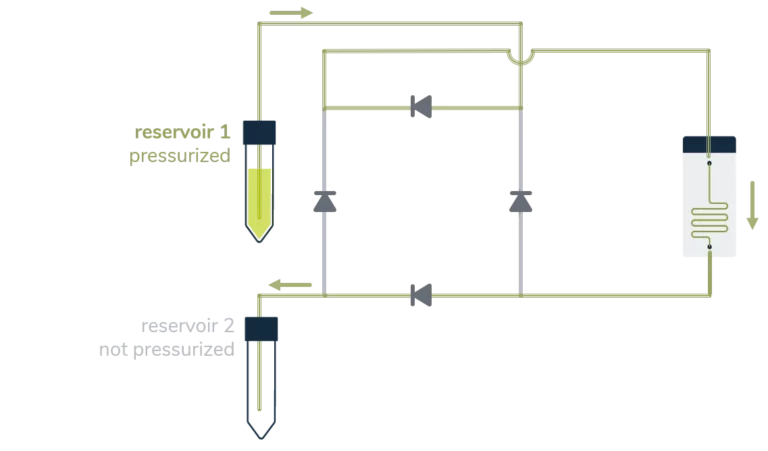
A great asset of a perfusion system is sustaining a constant unidirectional flow through a microfluidic chip while moving a limited volume of liquid back and forth between two reservoirs, as seen below.
From reservoir 1 to reservoir 2:
From reservoir 2 to reservoir 1:
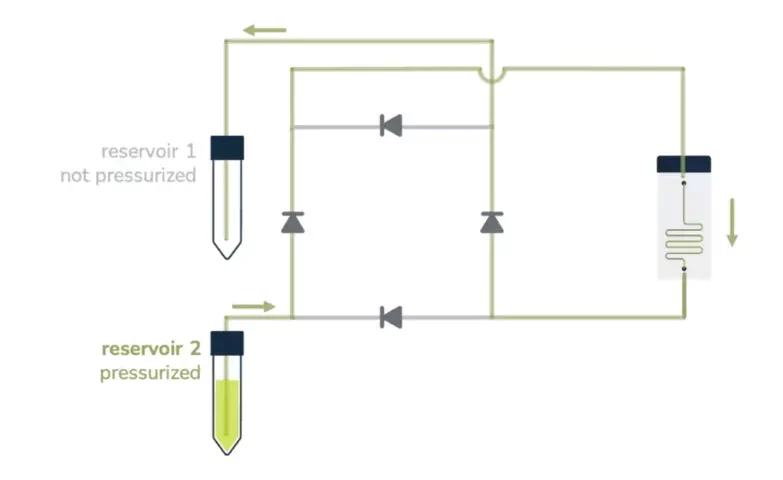
Ensuring that no air passes through the microfluidic chip is, most of the time, a significant concern in perfusion experiments and a tricky part of automating the system over a long period. It essentially relies on finding a consistent way to switch alternatively between the two reservoirs before one gets empty.
Usual solutions and limitations
Usually, it is achieved by estimating the time each reservoir needs to get empty based on the experiment’s flow rate and the system’s fluidic resistance. The system is then periodically switched from one configuration to the other for the duration of the experiment.
This way of automating the system looks neat in theory but is not very robust regarding the experiment. For instance, if the fluidic resistance of the circuit changes over time, or if it is different from reservoir A to reservoir B compared to from reservoir B to reservoir A (e.g., because of clogging), one of the reservoirs will get emptier after each switching period until it ultimately gets empty and air is injected in the chip.
A secured way to automate
This platform has a fail-safe mechanism that allows you to control the reservoir level and choose the minimal volume that will trigger the switch for each reservoir. This way, the perfusion system becomes insensitive to the imbalance between the two recirculation paths and ensures that air doesn’t alter the experiment.
Moreover, combining this system with a pressure-driven flow controller paired with a flow sensor and using a feedback loop on the flow rate will allow you to achieve an automated and fail-safe stem cell culture experiment at a constant flow rate.
A long-term stem cell culture platform
Stem cells can be perfused in a microfluidic chip for several days with a minimal medium volume using the stem cell culture platform. This platform is based on a pressure-driven flow controller, a recirculation bridge, and two-level sensors to achieve that. The specifications of the microfluidic level sensors can be found here.
With these additional features, the success of your microfluidic perfusion system is no longer dependent on the stability of your system’s fluidic resistance. In other words, you don’t need to worry about air entering your lines and damaging your cell culture due to fouling or clogging of the tubing!
In summary, the stem cell culture platform comprises the following instruments:
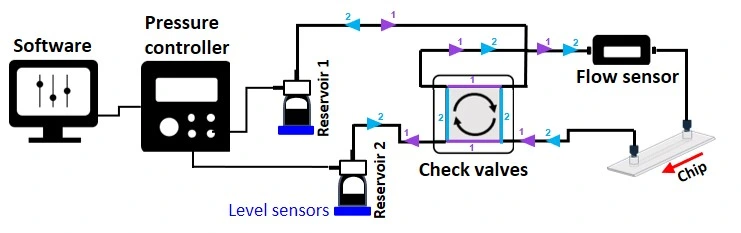
Stem cell culture platform includes:
Flow sensor (Galileo, MIC)
Software (Galileo user interface)
Recirculation bridge (check valves)
Level sensors
Pressure-driven flow controller
Several Schott bottles
Tubings and fittings
User guide
Microfluidic chip
Why use microfluidics for stem cell culture?
Culturing stem cells in microfluidics brings critical advantages in comparison to classical methods [1, 6]:
- Decrease experiment costs by reducing the amount of reagent used.
- Physiological shear stress is applied to the cells.
- Several solutions and physical factors can be controlled simultaneously with high precision.
- Even long experiments can be easily automated and don’t require human intervention.
- Improved oxygen and nutrition supply for cells.
- Better reproducibility and uniformity.
- Easily inject precise volumes of different drugs or compounds.
Thus, these advantages make microfluidics the best solution to perform stem cell culture and drug screening.
References
1. D. Van Noort, S. M. Ong, C. Zhang, S. Zhang, T. Arooz, and H. Yu, ‘Stem cells in microfluidics’, Biotechnol. Prog., vol. 25, no. 1, pp. 52–60, Jan. 2009.
2. R. Yoshimitsu et al., ‘Microfluidic perfusion culture of human induced pluripotent stem cells under fully defined culture conditions’, Biotechnol. Bioeng., vol. 111, no. 5, pp. 937–947, 2014.
3. P. Samal et al., ‘Grow with the Flow: When Morphogenesis Meets Microfluidics’, Adv. Mater., vol. 31, no. 17, p. 1805764, Apr. 2019.
4. N. Abdolvand, R. Tostoes, W. Raimes, V. Kumar, N. Szita, and F. Veraitch, ‘Long-Term Retinal Differentiation of Human Induced Pluripotent Stem Cells in a Continuously Perfused Microfluidic Culture Device’, Biotechnol. J., vol. 14, no. 3, p. 1800323, Mar. 2019.
5. I. R. Suhito, Y. Han, J. Min, H. Son, and T. H. Kim, ‘In situ label-free monitoring of human adipose-derived mesenchymal stem cell differentiation into multiple lineages’, Biomaterials, vol. 154, pp. 223–233, Feb. 2018.
6. K. I. W. Kane et al., ‘Automated microfluidic cell culture of stem cell derived dopaminergic neurons’, Sci. Reports 2019 91, vol. 9, no. 1, pp. 1–12, Feb. 2019.
7. J. Zhang, X. Wei, R. Zeng, F. Xu, and X. J. Li, ‘Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip’, Futur. Sci. OA, vol. 3, no. 2, pp. 187–2056, May 2017.
Application of stem cell culture in microfluidics
Stem cell culture can be used to keep stem cells in their undifferentiated state or to induce differentiation. Both cases require precise control of the cell culture microenvironment because stem cells are susceptible to external cues [1].
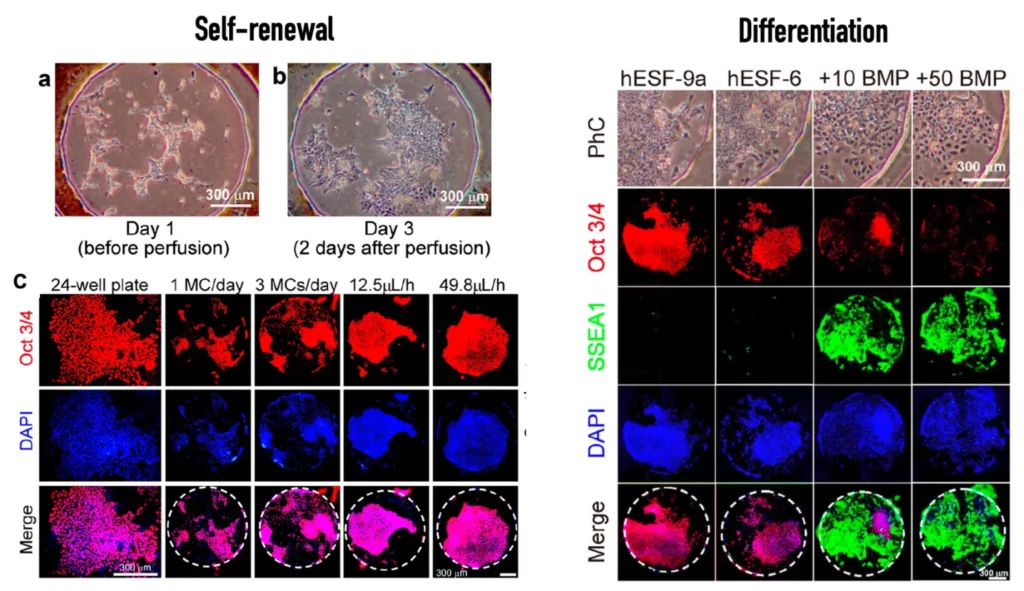
Yoshimitsu et al. [2] demonstrated the added value of microfluidics for stem cell culture by assembling a microfluidic platform with a custom culturing chamber and employing it to culture stem cells for self-renewal and differentiation. For that, the authors used known biomarkers to characterize each state.
References
1.D. Van Noort, S. M. Ong, C. Zhang, S. Zhang, T. Arooz, and H. Yu, ‘Stem cells in microfluidics’, Biotechnol. Prog., vol. 25, no. 1, pp. 52–60, Jan. 2009.
2. R. Yoshimitsu et al., ‘Microfluidic perfusion culture of human induced pluripotent stem cells under fully defined culture conditions’, Biotechnol. Bioeng., vol. 111, no. 5, pp. 937–947, 2014.
Customize your pack
Our instruments are compatible with standard commercialized chips from different brands.
Our Packs can be modified depending on your specific needs. In this light, our microfluidic specialists will advise you on the best instruments and accessories depending on your needs and will accompany you during the setup of the microfluidic platform.
Frequently asked questions
How can we help your experiment?
This pack is in beta testing phase. So, although the instruments are not fully industrialized, we can provide extensive support as part of our beta testing program. Get in touch to see if you are eligible.
Can a pack be customized based on my specific application?
Yes! Our experts will establish which instruments are best suited for your application, such as the type of flow sensor or the number of flow controller channels you need to perform your experiment. Contact us using the “talk to our experts” green button above.
Can I buy individual instruments?
Our instruments are in beta testing phase and can be tested as a pack or individually, so get in contact with our team to know how our beta testing program works.




