Cell perfusion in a cross flow membrane chip
Introduction
Microfluidics introduces the ability to flow medium continuously over cells in culture in a microfluidic chip. In addition to tight control of nutrients, pH and temperature, the flow rate and shear stress can be precisely controlled to mimic mechanical stimuli that cells experience in the body.
The choice of microfluidic chip can further complexify the microenvironment and add to the physiological relevance of the in vitro cell culture model. Cross-flow chips with multiple channels separated by a porous membrane barrier are a powerful design that enables the formation of cell interfaces for organ-on-chip models, and transport and migration assays.
This application note demonstrates the seeding and perfusion of U-251 MG human glioblastoma astrocytoma cells in the upper chamber of the cross-flow membrane chip Fluidic 480 (microfluidic ChipShop GmbH).
Applications
- Organ-on-chip barrier models of molecular transport across an interface:
- gas exchange, air-liquid interface (e.g. lung-on-a-chip model)
- transport and uptake of molecular components such as nutrients, toxins, pharmaceuticals (e.g. gut-on-a-chip, mother-fetal barrier, blood-brain barrier models)
- metabolism (e.g. liver-on-a-chip, multi-organ-on-chip models)
- filtration (e.g. kidney-on-a-chip model)
- Molecular gradients for transport assays
- Migration assays in response to angiogenic or other test molecules
- Dilution processes
- Buffer exchange
- Liquid-liquid interfaces
- In-line filtration
Experiment setup

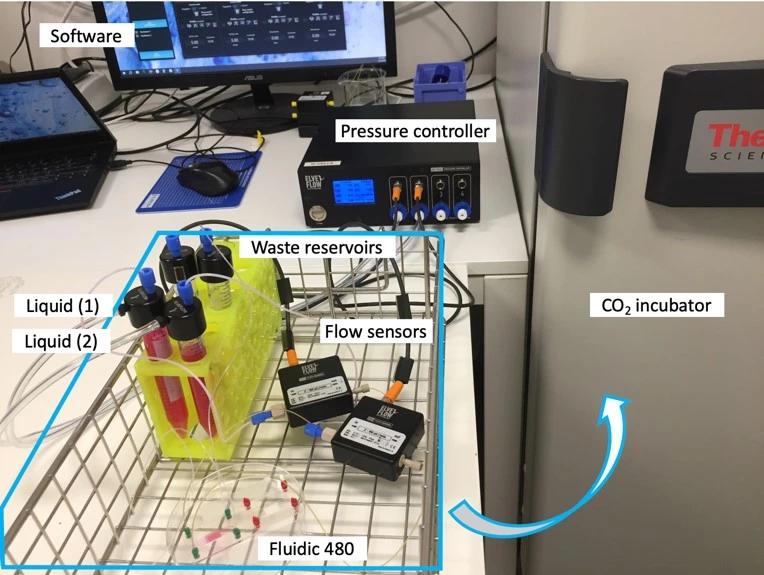
Flow controller OB1 (Elveflow)

Flow sensor (Elveflow)
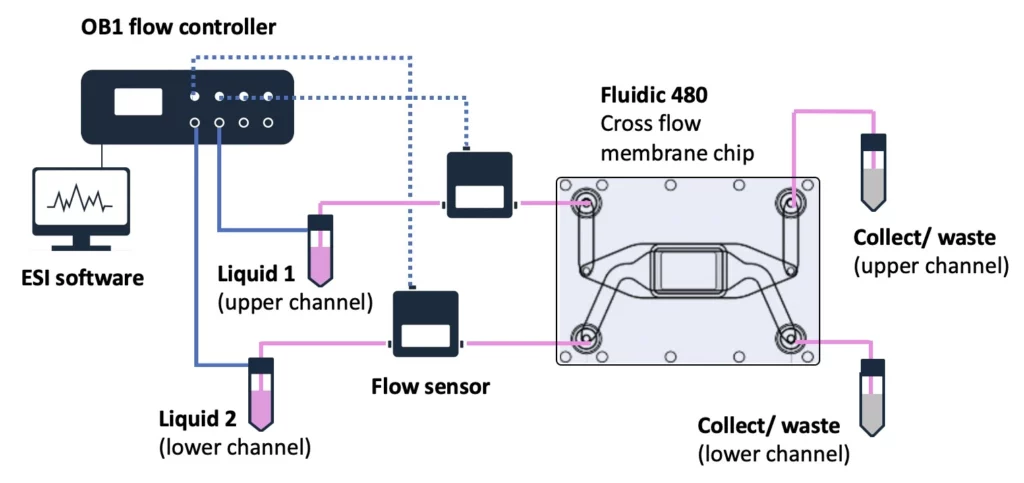
Materials
Hardware:
- OB1 MK4 flow controller (Elveflow) with two 0-2000 mbar channels
- Flow sensors, 2x MFS3 (Elveflow) (tropicalized, if to be used in the CO2 incubator)
- Tubings (PTFE, 760 μm and 1/16” outer diameter; OD), fittings, and reservoirs
- 2x 40 cm of 175 μm inner diameter (ID) microfluidic resistance (with 1/32” OD)
- Cross-flow membrane chip from microfluidic ChipShop GmbH, Fluidic 480
- Laminar flow hood
- CO2 Incubator
Chemicals:
- DMEM ([+] 4.5g/L D-Glucose; Gibco)
- Penicillin/ Streptomycin, 1% (10,000 U/ml Penicillin, 10 mg/ml Streptomycin; PAN Biotech)
- FBS Good, 10% (0.2 μm sterile filtered; PAN Biotech)
Software:
- ESI software
Design of the chip
The cross-flow membrane chip (Fluidic 480, microfluidic ChipShop GmbH) has two stacked chambers, interconnected by a semipermeable membrane. There are two units per slide. The chip is intended to be seeded with cells on either side of the central porous membrane to create an interfacial barrier layer from multiple cell types. However, it may also be seeded on just one side for filtration, transport, migration and other molecular gradient assays.
Flow can be added to the upper, lower and/or both chambers, depending on the assay needs and sensitivity of the cell types used. If a tight cell monolayer is formed on the entire membrane surface then different flow rates can be more easily maintained in the two chambers. If a cell monolayer is not complete, the two different liquid flows will equalize at the point of the membrane due to its porosity, and the exchange of liquids between the chambers will occur more rapidly.
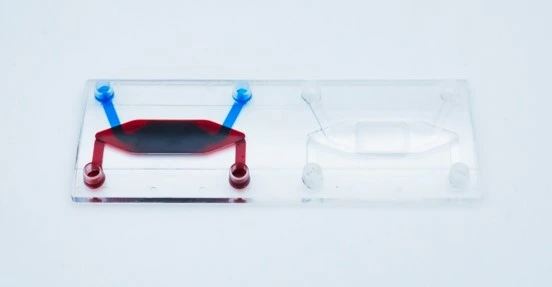
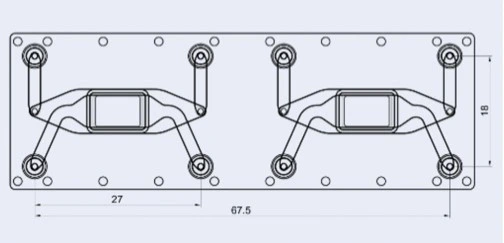
Details of Fluidic 480
| Fluidic 480 | Features |
| Interface type | Female Mini Luer |
| Chamber volume | Upper chamber: 87.5 μl Lower chamber: 61.5 μl |
| Membrane surface area | 0.36 cm2 |
| Membrane pore size | 0.2 μm |
| Chip material | Topas |
| Surface treatment | Hydrophilized |
| Lid thickness | 140 μm |
Quick start guide
Instrument connection
1. Connect your OB1 pressure controller to an external pressure supply using pneumatic tubing, and to a computer using a USB cable. For detailed instructions on OB1 pressure controller setup, please read the “OB1 User Guide”.
2. Connect the flow sensors to the OB1. For details refer to “MFS user guide”.
3. Turn on the OB1 by pressing the power switch.
4. Launch the Elveflow software. The Elveflow Smart Interface’s main features and options are covered in the “ESI User Guide”. Please refer to the guide for a detailed description.
5. Press Add instrument \ choose OB1 \ set as MK4, set pressure channels if needed, give a name to the instrument and press OK to save changes. Your OB1 should now be on the list of recognized devices.
6. OB1 calibration is required for the first use. Please refer to the “OB1 User Guide”.
7. Add the flow sensors: press Add sensor \ select flow sensor \ analog or digital (choose the working range of flow rate for the sensor if you have an analog one), give a name to the sensors, select which device and channel the sensor is connected to and press OK to save the changes. Your flow sensor should be on the list of recognized devices. For details refer to “MFS user guide”.
8. Open the OB1 Window.
Chip preparation, filling and seeding
1. You can choose between different polymer materials. Generally, the best material for cell culture due to its biocompatibility is PS. However, COC (Topas) also works well. These materials are naturally hydrophobic, but the integrated membrane is hydrophilized and already suitable for adherent cell lines. For specific experiments, the cross-flow membrane chip is also available as fully hydrophilized, improving fillability and the adhesion of static cells to the material.
2. Prepare cell suspension as per standard protocols. Ensure all cell clumps are gently but well dissociated and count carefully. Use the suspension immediately.
3. Seed cells on the membrane in the upper channel at a cell density of 1 x 106 cells/mL. (Note: this protocol describes adding cells to the upper chamber only. To add cells to both chambers, start by seeding the lower chamber; see Optional Step 5).
First, fill the bottom chamber of Fluidic 480 with medium using a pipette positioned directly into the small hole at the base of the inlet, using one smooth movement of the plunger. Take care when removing the pipette from the hole to not create back pressure that may pull air from the upper chamber through the porous membrane.
Then, fully fill the top chamber with cell suspension by pipette the same way.
4. Cover inlets/ outlets and leave 4-16 h in the CO2 incubator for cells to attach. To ensure channels do not dry out during incubation, secure the chip in a covered petri dish containing a lid of water to keep the environment moist. An unused chip on the slide can be kept sterile with Mini Luer plugs.
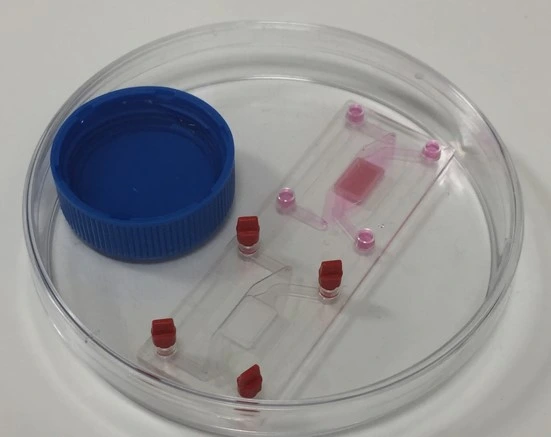
Figure: Incubation for cell attachment. The chip was secured in a closed petri dish containing a small reservoir of water to humidify the environment.
5. [Optional] To seed cells on the membrane in both chambers (or in the lower chamber only): Start by adding cell suspension to the lower chamber. Then fill the upper chamber with medium and plug all interfaces (prefer low volume displacement Mini Luer plugs to protect against air entry upon their removal). Incubate the chip inverted for 4-16 h for cells to attach.
After cells are attached to the lower membrane surface, add cell suspension to the upper channel using a pipette. Incubate the chip for another 4-16 h for cells to attach.
Once cells are attached to the cross flow membrane chip, the set-up can be prepared.
Set-up preparation
1. Connect the reservoir caps to the OB1 with pneumatic tubing.
2. Connect each reservoir to one flow sensor with 1/16” OD tubing. Add 40 cm of 175 μm ID microfluidic resistance tubing (with 1/32” OD) to the outlet of the flow sensors.
3. Add a Mini Luer tube tuck connector to the free ends of the microfluidic resistance (they will be connected later to the inlet of the chip).
4. Connect a Mini Luer tube tuck connector to one end of a piece of 760 μm OD tubing (destined for the chip outlet, lower channel) and secure the other end in a waste reservoir. Repeat, to collect waste from the upper channel.
Chip connection to fluidic circuit
1. Connect the lower channel reservoir to the system and purge the line until a droplet is visible at the outlet of the resistance (the chip is not attached yet).
2. Connect the resistance to the inlet of the lower chamber of the chip. Then connect the outlet tubing to the outlet of the lower chamber.
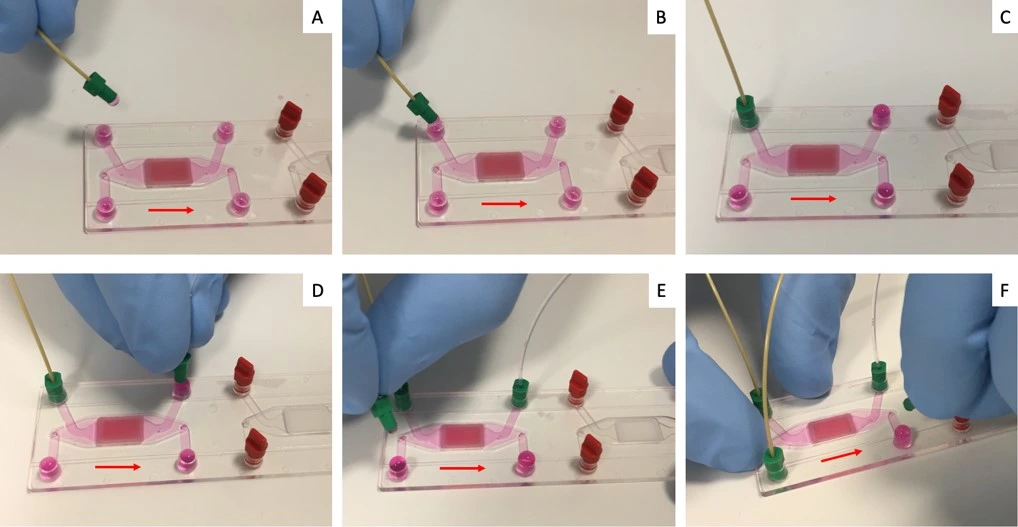
3. Repeat steps 1 and 2 above to purge tubing and connect the fluidic circuit to the upper channel of the chip.
Experiment
1. Flow at desired flow rate for desired time (e.g., 5 μl/min for 16 h).
2. Analyze (e.g., image cells in the chip).
Results
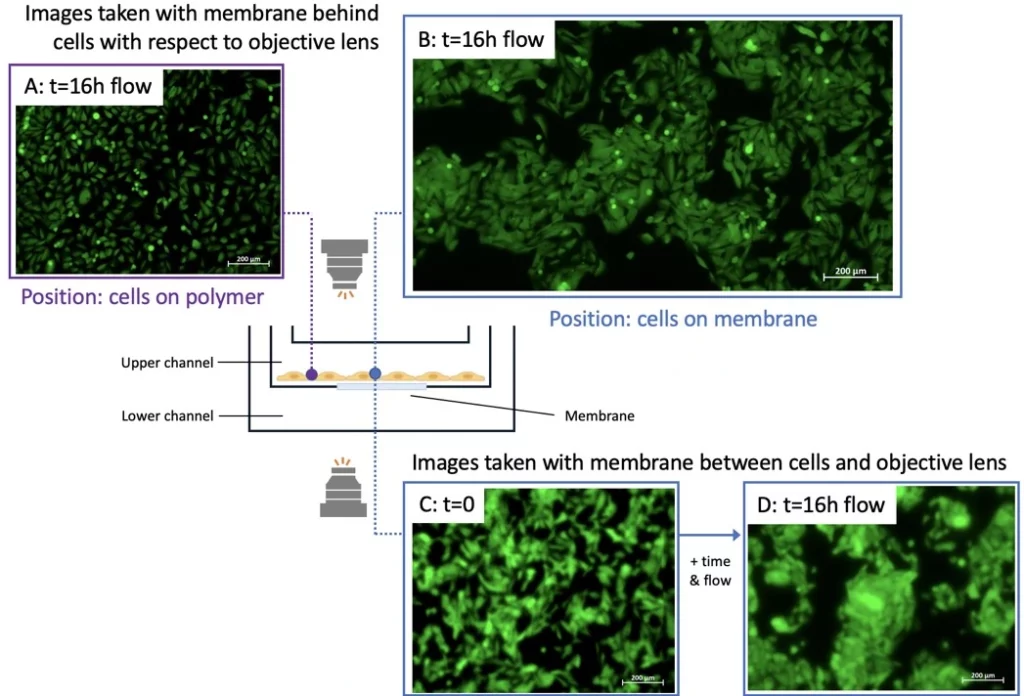
Cells were seeded in the upper chamber of the cross flow membrane chip and perfused for 16 h. No flow was set in the lower channel. Cells were imaged from below the membrane (i.e. with the chip upright) and after inverting the chip using an Axio Observer inverted microscope (Figure). Cells were imaged at two positions: on the membrane (position indicated by blue circle) or on the polymer surface (i.e. off the membrane; purple circle).
While it was difficult to image cells through the membrane (i.e. when the membrane was between the objective lens and the cells), cells were clearly imaged when the membrane was positioned behind the cells (i.e. when the cell layer was between the objective and the membrane). Cells attached to the polymer surface were easily imaged regardless of chip orientation with respect to the objective.
The chip membrane may absorb stain if cell staining is performed in-chip, especially for assays that do not use a confluent cell monolayer, increasing the background signal. In this case, consider pre-staining cells before seeding with a live cell stain if imaging is required, or use the chip for applications that collect and analyze supernatant as an off-chip readout.
Acknowledgements
This application note is part of a project that has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 101036702 (LIFESAVER).

