Phenotypic variation by single-cell analysis: TREATABLE
Author
Christa Ivanova, PhD
Publication Date
October 30, 2021
Keywords
tuberculosis therapy
single-cell analysis
phenotypic variation
persistent infections
drug resistance
drug chemistry
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
The goal of project TREATABLE is to target phenotypic variation to enhance tuberculosis therapy.
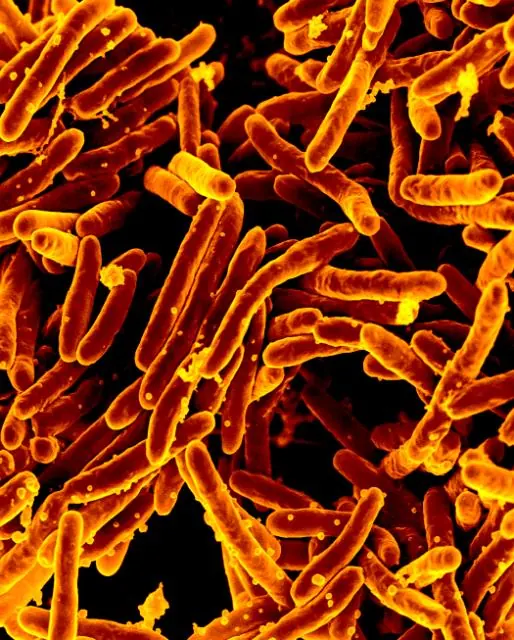
In 2020, tuberculosis was responsible for 1.5 million deaths worldwide, making it the second leading infectious killer. The United Nations aims to reduce tuberculosis incidence and mortality by 2030 drastically.
Even though the incidence steadily falls by about 2% yearly, it is only half as much as needed to reach the target.
An aggravating factor lies in the increase in drug-resistant Mycobacterium tuberculosis (Mtb, the pathogen of tuberculosis).
Tuberculosis is a curable disease, which requires a combination of four drugs with a cure rate of 85% for the drug-sensitive form but a more complex medication for the drug-resistant strains, lowering the cure rate down to 50%.
Due to its clonal population structure, Mtb’s ability to form phenotypic variants relies not only on genetic mutations but also on non-genetic bases, which can be induced or enhanced by a complex interplay with its environment.
We hypothesize that this phenotypic variability is key in the persistence of Mtb and is an effective leverage to develop new therapeutics.
Even though the study of tuberculosis persistence is evolving, it is essential to go beyond observations averaged over a population and to foster studies on sub-populations on which the mechanisms of phenotypic variation can be isolated.
Targeting phenotypic variation by single-cell analysis: project description
TREATABLE project (Target phenotypic variation to enhance tuberculosis therapy) aims to use the partners’ skills in cell biology, microfluidics, and drug chemistry to assess the effectiveness and the activity of novel drugs on drug-resistant Mtb single-cells.
An existing microfluidic platform will be upgraded through a large-scale, robust, and fast fabrication process and the addition of media controllers (pH and atmosphere) to enable a stable multiphasic time-lapse imaging of Mtb single cells exposed to a collection of compounds. The compounds will be selected from an extensive chemical library based on their putative anti-persistence ability.
Synergetic effects with existing drugs will be explored. The understanding gained on the mechanisms of phenotypic variation of Mtb might also be transposed to other persistent infections and pave the way towards the elaboration of new therapies.
Microfluidics will play a critical role in parallelizing the experiments, allowing us to explore and compare a more significant amount of chemicals. It will also be vital in achieving a subpopulation observation on which phenotypic variation mechanisms can be observed.
We believe this project will contribute to a breakthrough in further developments in tuberculosis therapeutics and the understanding of single-cell dynamics.
The consortium gathers well-established partners from France with the Institut Pasteur (coordinator), the Université de Caen Normandie on the academic side, and the Microfluidics Innovation Center and BlackHole Lab on the industrial side.
Related content
In the light of this project, we have published a review about materials and fabrication techniques of microfluidic chips.
Funding
This project has received funding from the French Agence Nationale de la Recherche (ANR) with project ID: ANR-21-CE15-0045 (TREATABLE).
Start date: 1 January 2022
End date: 31 Dec 2025

Check our Projects
FAQ – Phenotypic variation by single-cell analysis: TREATABLE
What is the key question of TREATABLE?
TREATABLE posits the idea of drug-tolerant subpopulations of Mycobacterium tuberculosis (Mtb) that do not appear genetically dissimilar to each other but act in different ways can be profiled and, as a result, targeted to enhance the rate of cure. Instead of average readouts in a culture, the project targets single-cell dynamics to identify rare and persistent phenotypes that are generally eluded by bulk assays.
So what is the significance of phenotypic variation on tuberculosis therapy?
Cells may even enter transient states in clonal Mtb populations, thereby deactivating antibiotic action. These non-genetic variants contribute to persistence and relapse, and they end up being even more problematic as drug resistance increases. TREATABLE will reveal these variants at cell-by-cell resolution of behavior and identify compounds that neutralize them, alone or in combination with drugs already available on the market to treat TB.
Which microfluidic methodology is in use?
The consortium is enhancing an existing microfluidic platform for long-term, multiphase time-lapse imaging of individual Mtb cells in specifically controlled media, pH, and gas atmospheres. Massively parallelized chip layouts can be used to compare many conditions simultaneously, enabling robust comparisons within a curated library of anti-persistence compounds.
What does multiphasic time-lapse maintain individual bacteria in stable micro-niches, and then apply to practice?
It allows scientists to maintain individual bacteria in stable micro-niches and then perturb them in stages (e.g., pre-stress – drug pulse – recovery) with physiological control. It is that design that allows the capture of slow-growing or dormant phenotypes, which would otherwise be diluted or averaged out in flasks.
What is the method of choice and screening of candidate compounds?
A large library of chemicals is down-selected to compounds based on predicted anti-persistence activity. Single-cell results, such as growth arrest, delayed division, and timing of death, are then measured on the platform and screened for synergies with conventional four-drug TB regimens. Hits that kill tolerant subpopulations repeatedly are of priority to be followed up.
What is the numerical issue in global health that this is addressing?
Tuberculosis resulted in approximately 1.5 million deaths in 2020, second in the list of infectious killers. The reduction has been about 2 percent per year, well short of what is needed to meet the United Nations’ 2030 targets. The common initial treatment regimen treats about 85 percent of TB that is sensitive to the drugs, with the results possibly dropping to about 50 percent in drug-resistant instances. Such loopholes prompt the development of effective plans against chronic cells.
Who are the engaged parties, and what can they bring?
A team of staff located in France is brought together in the project: Institut Pasteur (coordination, tuberculosis biology), Université de Caen Normandie (academic research), Microfluidics Innovation Center (microfluidic engineering, automation, valorization), and BlackHole Lab (industrial microfabrication). This combination brings together high-technology biology and scaffoldable, scalable microfluidic tooling for screening applications.
What does the schedule and budget appear to be?
The project TREATABLE has project ID ANR-21-CE15-0045 and is financed by the French National Research Agency (ANR). It will run from 1 January 2022 to 31 December 2025. The latter range includes platform industrialization, assay validation, and multi-compound testing against resistant Mtb single cells.
What is the specific value that MIC brings to the consortium?
MIC develops the high-throughput microfluidic cultures, maintains the environment (pH, atmosphere), and simplifies the fabrication such that chips can be generated in large quantities- essential in statistically powered single-cell screens. In addition to the laboratory activity, MIC facilitates proposal writing and technology transfer to make sure that the validated assays become powerful instruments. Introducing a specialist SME such as MIC into a consortium generally boosts feasibility and exploitation planning, internal experience of success rate of proposals, proposal success rates seem to go up about two-fold in cases where we are integrated at an early stage.
Can these techniques be used in other infections?
Yes. The identical single-cell, controlled-microenvironment rationale can be applied to all other persistent infections and hard-to-control subpopulations – any environment where non-genetic tolerance leads to treatment failure. The modularity of the platform (media control, gas composition, multiple lanes, parallel) makes it easy to transfer to new pathogen or host-pathogen models.
What will be considered as proof that attacking phenotypic variation is effective?
Two coinciding signals: (i) quantitative loss of tolerant subpopulations in repeat single-cell assays, and (ii) reproducible range of synergy with current drugs that causes kill-time distributions to shift left (faster clearance) and the variance of kill-time distributions to decrease (fewer late survivors). The metrics are expected to be included in the project design.
How might possible partners or TB scientists get involved?
If you are considering persistence, tolerance, or hard-to-treat infections, the MIC team can assist with scoping a microfluidic assay, prototyping the chip, and co-authoring competitive European proposals. We provide regular support for Horizon Europe applications and industrialize custom devices to ensure that validated biology does not languish at the proof-of-concept stage.