Plug-n-play tool-kit of organ-on-chips: PTOoC
Author
Veerendra Kalyan Jagannadh, PhD
Publication Date
September 28, 2019
Status
Keywords
micro-physiological systems
Organ-on-chip
drug testing
bioengineering
physiological relevance
preclinical screening
interdisciplinary biomicrofluidics
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
About 20% of kidney injuries acquired during hospitalization are due to drug-induced nephrotoxicity, which the current preclinical models failed to predict.
There is an imminent need for inexpensive drug-development models with high physiological relevance using organ-on-chips.
Plug-n-play toolkit for organ-on-chips: introduction

Understanding the human physiological response to newly developed drug molecules is critical in drug discovery and development. Drug discovery heavily relies on two approaches: well-plate-based cell culture and animal model-based testing.
These approaches fail to provide the required early-stage predictive insight, as they lack the required degree of physiological relevance.
The demand for testing systems with high physiological relevance while being cost-effective has led to the development of Organ-on-Chips (OoC). In contrast to conventional cell culture-based methods, organ-on-chips enable control over various physical, chemical, physicochemical, and biochemical cues required to recapitulate the minimum functional unit of a human organ.
Plug-n-play organ-on-chips: more physiologically relevant models
In recent years, there has been significant progress in organ-on-chips with the development of several MPS micro-physiological systems that enable in vitro modeling of different human body organ systems. However, organ-on-chips have yet to cross specific key barriers for them to become the gold-standard method for drug testing.
The current state-of-the-art organ-on-chips suffer from low standardization levels and low throughput workflows compared to their conventional cell culture-based counterparts.
Further, the functional scaling of organ sizes and vascular flow warrants a significant design constraint in developing organ-on-chips. So far, the design has followed the approach of identifying the minimal functional/physiological aspect of a given organ and implementing the various physiological cues, using various micro-actuation and microfluidic techniques.
This has led to several varied approaches to the design problem, with very little focus on modularity and standardization. The low level of standardization makes it extremely difficult to compare studies performed by different research groups.
Moreover, using current commercially available organ-on-chips requires at least some basic training for non-experts in microfluidics. Very few systems exist which can enable a simple and efficient plug-and-play level of usability.
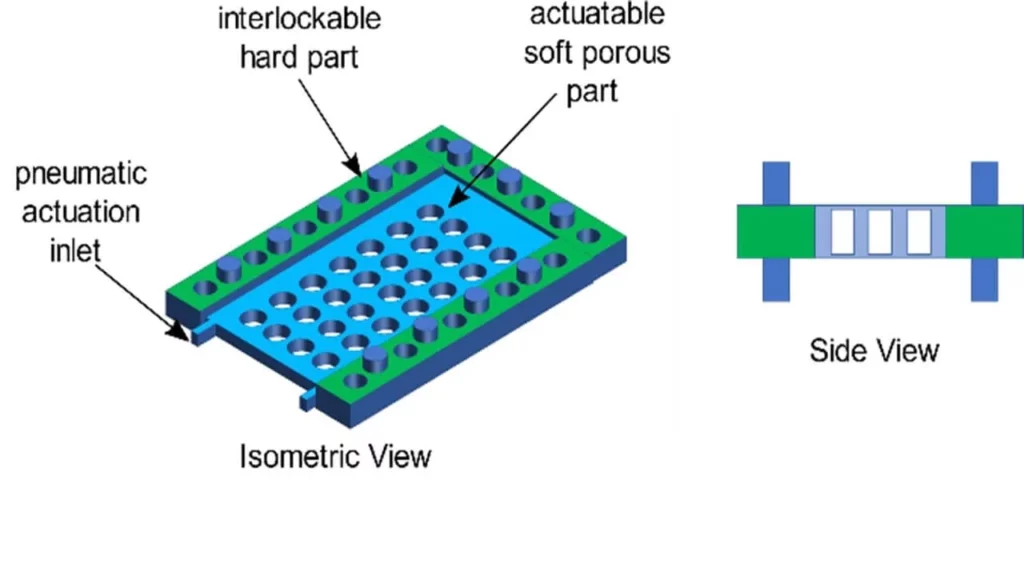
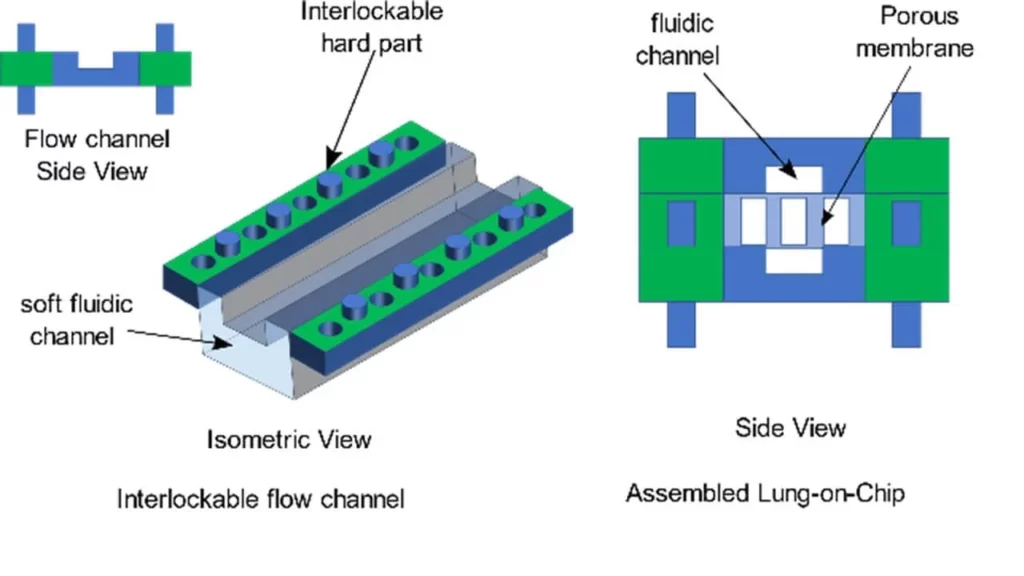
Plug-n-play Organ-on-chips: project description
To tackle the problems of standardization and ease of use, we propose a modular approach to design and fabricate organ-on-chip systems.
In this project, we propose to develop a standardized consumable parts library that can assemble any organ-on-chip system as the user requires.
Each part of the library would enable a specific physicochemical stimulus/structure warranting its use in designing a particular type of organ-on-chip. The consumable parts library would enable quick design and rapid prototyping of organ-on-chip systems while instilling a high level of standardization in the domain.
The developed parts library would be compatible with the existing world-to-chip Elveflow microfluidic interfaces. This creates an inexpensive, complete, easy-to-use toolkit for organ-on-chip-based drug testing.
Organ-on-chips require exact control over several experimental parameters like flow rate, pressure, etc.
In conjunction with the used sensors and controllers, the developed parts library would provide the required control and programmability with a high degree of precise control over experimental parameters.
Microfluidic calculator
During this project, we developed the Elveflow microfluidic calculator. Now, you can choose the perfect instrument calibrated for your needs, determine your flow rate, the pressure you need to apply, the best tubing resistance length for your setup, wall shear stress for biology applications, cell culture, and many more.
This project has received funding from the European Union’s Horizon 2020 MSCA-IF under grant agreement No 840231 (PTOoC).
Researcher

Dr. Veerendra Kalyan Jagannadh
Research Associate
- PhD in in Applied Physics (Indian Institute of Science, India)
- Bachelor of Engineering in Electronic Instrumentation (Birla Institute of Technology & Science-Pilani (BITS-Pilani), India)
Areas of expertise:
Microfluidics, Optics, Digital Image processing.


Check our Projects
FAQ – Plug-n-play tool-kit of organ-on-chips: PTOoC
What specific issue was the PTOoC project attempting to address?
In organ-on-chip (OoC), brilliant prototypes often get lost because each device has its own connectors, footprint, and flow logic. PTOoC addressed this directly by suggesting a standard, modular library of consumable parts so researchers can build chips and peripherals much like building blocks, rather than having to rediscover the interface.
What is a tool-kit that can be plugged and played in this case?
Imagine a system of inter-lockable hybrid microfluidic components which are hard or soft, microfluidic bases, gaskets, lids, fluidic ports, sensing inserts, and flow adapters which can or cannot open or close in predictable patterns. Every component has a particular function (shear, mixing, barrier culture, perfusion, sampling) and the geometry is limited in a way that it is interoperable between laboratories.
So what was actually delivered in the project?
A design system and a library of parts that allows quick assembly of various OoC layouts based on a limited number of standardized parts. The resulting effect: accelerated time-to-market, reduced maintenance, and fewer one-off custom designs. Practically, scientists were able to develop an idea to an experimentable chip design within hours as opposed to weeks.
What is the relationship between PTOoC and the standardization efforts in Europe?
The project is in line with the greater drive of shared dimensions, ports, and interfaces in OoC. PTOoC simplifies the validation of assays between sites, and harmonises with new European standardisation roadmaps of OoC, by addressing the issue of the problem of how things connect, that is, the dimensions, clamping pressures, flow ranges.
What does this assist in regarding reproducibility and tech transfer?
You eliminate a significant source of variability when there is overlap between the seals, ports, and cross-sections of the channels in two labs. With the protocols made portable, a typical use of them is that it became possible to set flow to X, clamp to Y, and use port P1 to sample, and the industrial partners are able to implement the design quicker since the mechanical interfaces are already established.
What is compatibility with the pumps and sensors that I already have?
PTOoC intentionally designed the common footprints and adapters such that you may route to syringe pumps, peristaltics or pressure-driven controllers and insert standard probes (pressure, flow, TEER-style electrodes) through known ports. Stated another way: do as little custom machining as possible, do as much re-use of your bench as possible.
My circuit is a multi-organ, is the toolkit taking cross-talk and scaling into account?
Yes, level of fluidics and form-factor. You have the ability to partition circuits having known hydraulic resistances, insert or delete modules, and isolate tissue niches together sharing a vascular channel. Rules concerning scaling (surface to volume, residence time) are addressed by selecting among a small set of channel heights and widths which are mapped to physiological shear ranges.
What is the place of Microfluidics Innovation Center (MIC)?
MIC also helped in the design side of the work-flow, manufacturable interfaces and fast prototyping. Our niche is to research-grade ideas and convert them into strong assemblies: consistent seals, pristine centering and components that will resist being used in normal cell-culture. We are also the ones that enable consortia to bridge the gap between the hardware and automation (closed-loop perfusion, imaging-ready carriers).
Is MIC interested in being a part of my Horizon proposal and industrializing the prototype with me?
Yes. Being an R&D SME in the field of microfluidic engineering, we regularly become members of European consortia (i) to engineer designs into prototypes that can be shipped, (ii) to write realistic work plans and risk registers, and (iii) to prepare scale-out. As practice demonstrates, when an able SME is involved to provide integration and prototyping, it materially enhances the competitiveness of the proposal; in our experience, the presence of MIC in the programme has increased success rates by nearly 2:1, on average, compared to published programmes. If your call hits OoC, automation, or fast microfabrication, we are ready to add value on the first day.