Multi-compartmental tumor-on-a-chip to study breast cancer: MTOAC
Author
Subia Bano, PhD
Publication Date
September 19, 2018
Status
Keywords
Tumor-on-chip
Organ-on-a-chip
3D hydrogel
breast cancer model
combination therapy testing
tumor microenvironment modelling
tumor metabolism
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Tumor on-a-chip device for drug screening of breast cancer: introduction
Breast cancer is one of the most fatal cancers for women all over the world. Even if significant progress has been made in treatments, drug distribution at the molecular level is not fully understood yet, and some treatments remain ineffective.
The use of a tumor-on-chip model could lead to a better management of this cancer.

Currently, cancer research is mainly based on studies performed either on 2D cell culture, which is not representative of real in vivo conditions, or on living animals, leading to ethical issues and questionable extrapolation to humans.
Organ-on-a-chip technology, particularly tumor-on-a-chip, allows us to mimic the cells’ microenvironment accurately, to study diseases’ mechanisms, or to test new drugs.
It offers a realistic animal-free alternative to better understand breast cancer mechanisms.
Multicompartemental tumor-on-a-chip: project description
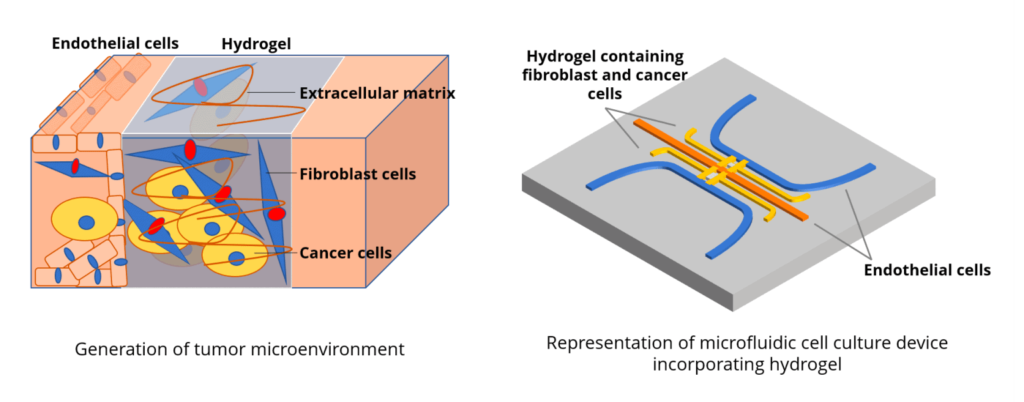
In this project, we will recreate the environment of breast tumors by surrounding tumor organoids with fibroblasts and endothelial cells, all coculturing in a biocompatible hydrogel.
In the tumor-on-a-chip model, the microenvironment of the cells will be carefully controlled with a pressure controller (Elveflow) combined with valves and actuators. We will test different combinations of anti-cancer drugs to study the existing synergies between them and better understand how they interact with the tumor.
This drug screening will allow the development of more effective breast cancer treatments.
Related content
Have a read here on the breast tumor-on-a-chip devices review written by Dr. Subia Bano.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 795754 (MTOAC project).
Researcher

Dr. Subia Bano
Research Associate
- Post-Doc at the University of Eastern Finland
- Post-Doc at the Institute of Genomics and Integrative biology (India)
- PhD in Biotechnology (Indian Institute of technology, India)
Areas of expertise:
Tissue engineering, Biomaterials, Drug Delivery, Tumor-on-chip.
Check our Projects
FAQ – Multi-compartmental tumor-on-a-chip to study breast cancer: MTOAC
What is MTOAC?
MTOAC is a study that developed a microfluidic tumor-on-a-chip to provide a superior in vitro model of breast cancer tumours. The chip enables the study of drug responses and tumor behavior in a 3D, multicellular microenvironment, rather than using standard 2D cell culture or animal models.
Why is MTOAC required - what is the problem it attempts to solve?
In vitro models (2D cell cultures) are not complex enough to mimic the dynamics of real tumour microenvironment, animal models are too expensive, and, more often than not, they do not reproduce the behaviour of individual human tumours.
MTOAC bridges this gap by creating a human-relevant, ethically grounded in vitro system that better mimics physiological conditions, thereby enhancing the predictive validity of drug screening and cancer biology.
What is the MTOAC chip - how is the platform used?
The chip mimics the microenvironment of a tumour by co-culturing cancer (breast tumour) cells with supporting cells (fibroblasts and endothelial cells) in a biocompatible hydrogel.
The microfluidic channel, using a pressure controller (e.g., Elveflow), valves, and actuators, regulates fluid flow, nutrient delivery, and microenvironmental conditions within compartments.
This configuration can be used to produce 3D tumour-like tissues, which permit the cell-cell interaction, tissue heterogeneity, gradients (e.g. oxygen, nutrients), and realistic responses to treatments.
What type of experiments or applications is MTOAC working on?
- Combining anticancer drugs (drug screening), to determine the potential synergies and the more effective combinations.
- Understanding tumor biology – The ways in which cancer cells communicate with stroma (fibroblasts), endothelium, and the extracellular matrix, which may influence tumour growth, invasion, and metastasis.
- A more human-relevant and 3D in vitro culture model between cell culture and in vivo (animal) models to decrease animal dependence and enhance translational relevance.
Why is the tumor-on-a-chip technique superior to the 2D model or animal models?
- More physiologically relevant microenvironment: 3D architecture, co-culture of multiple cell types, extracellular matrix, fluid movement are more physiologically-relevant microenvironments of in vivo conditions compared to flat 2D cultures.
- Ethical and cost advantages: No use of animals, and more controlled and reproducible experiments can be made, standardized, and scaled experiments.
- More realistic modelling of tumour heterogeneity and dynamics: The dynamics and gradients (oxygen, nutrients), cell-cell and cell-matrix interactions, multicellular architecture, and realistic drug diffusion/response can be studied in greater detail.
What was completed in the MTOAC project? What is the position?
The project has designed a multi-compartment microfluidic chip that supports the co-culture of breast cancer cells, fibroblasts, and endothelial cells within a hydrogel matrix. It also assessed the chip’s ability to restore 3D tumor-like structures under controlled flow, which prepares the way for drug screening and biological research.
What do you consider to be the possible shortcomings or obstacles of the MTOAC approach?
Since the system aims to recapitulate a more complex tumour microenvironment, it can be technically more challenging than simple cell culture: the design and control of fluidic flow, co-culture environments, hydrogel scaffolds, and reproducibility are not trivial. As well, although the chip is closer to in vivo conditions than 2D culture, it remains a simplified model of a full living organism – other elements of tumor biology (immune system interactions, whole-body pharmacokinetics) fall beyond its reach.
Is this a method that will completely substitute the use of animal models?
Not completely: though tumor-on-a-chip offers an attractive alternative to animal-free testing with numerous benefits, it is still a model. It can mimic a lot of aspects of the tumour microenvironment – cell types, architecture, flow, 3D structure – but cannot mimic the entire complexity of an organism (immune system, full pharmacokinetics, systemic interactions). The chip can be described as a potent supplementary resource applicable to mechanistic research and pre-clinical drug screening, though it does not fully replace all in vivo research.