Heart-on-chip for personalized medicine: CISTEM
Author
Christa Ivanova, PhD
Publication Date
January 14, 2018
Status
Keywords
Heart-on-chip
Lab-on-a-chip
mechanobiology
Duchenne muscular dystrophy
heart disease modeling
cardiac function monitoring
personalized medicine
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Duchenne muscular dystrophy is still poorly understood, and no efficient treatment is available. The promising heart-on-chip models might be a first step towards better managing Duchenne disease.
Heart-on-chip for personalized medicine: introduction
Duchenne muscular dystrophy occurs in 1/3500 children and leads in 60% of patients to the development of cardiomyopathy, a heart dysfunction, in the second decade of life.
This disease is still poorly understood, and no efficient treatment is available. The use of heart-on-chip models is promising for better management of Duchenne disease.
Organ-on-a-chip, which can mimic the cell microenvironment in a real organ, are animal-free promising systems for a deeper study of this rare disease and for screening potential new drugs.
However, the mechanisms of cardiomyopathy vary from one patient to another, demanding the development of personalized treatments adapted to each patient.
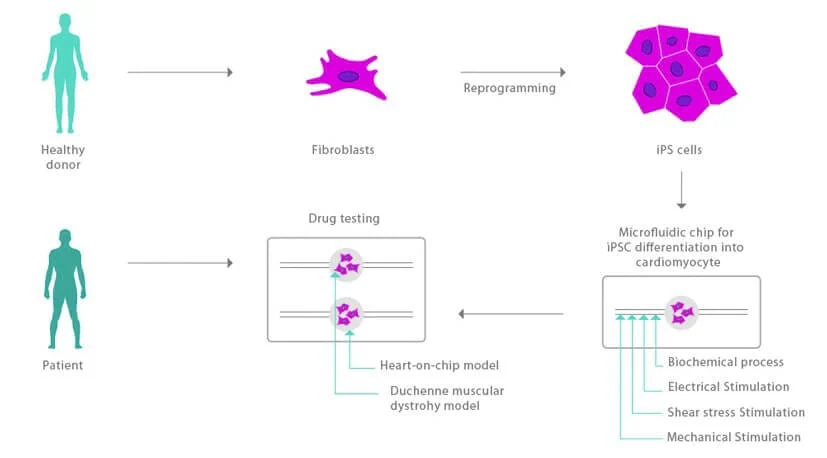
In the CISTEM project, we aim to develop a more precise and personalized heart-on-a-chip model; human-induced pluripotent stem cells are derived in a patient-matched manner in a microfluidic device.
This system will allow precise control of shear stress and electrical stimulation, mechanical strain, and surface morphology.
Heart-on-chip for personalized medicine: project description
We will design and fabricate this microfluidic chip to differentiate pluripotent stem cells at a microscale.
Elveflow pressure controller will handle fluids into this new heart-on-a-chip. Its fast and stable pressure control will allow the precise monitoring of the shear stress applied to the cells, ensuring the creation of biological conditions as closely as possible.
In addition, the possibility to easily configure complex functions for the pressure profile enables the reproduction of the physiological effect of a beating heart. This heart-on-a-chip will allow a better understanding of cardiomyopathy and personalized drug testing.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 778354 (CISTEM project).



Check our Projects
FAQ – Heart-on-chip for personalized medicine: CISTEM
What is the practical goal of CiStem?
To develop a microfluidic heart-on-a-chip that mimics human heart-beating, perfusion, response to drugs, and so on, such that clinicians and R&D departments can predict patient-specific response before it even comes to the clinic.
What is the difference between a heart-on-a-chip and a regular cell culture plate?
Three macro-changes: Continuous perfusion as opposed to stagnant media; mechanically-relevant cues (contractility and cyclic stretch at about 1-2 Hz); structured flow establishing physiological shear (usually 0.5-2 Pa in microchannels). Combined with this, it stabilizes the phenotype and reveals drug effects not visible on ordinary plates.
What is the source of tissue - primary cells or IPS cells?
It consists of a platform based on human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), and can be co-cultured with endothelial cells and fibroblasts. The iPSC pathway enables patient-comparable chips with the same genome, risk profile, etc., and personalized pharmacology is a realistic, not aspirational, goal.
What readouts are measured on-chip?
Electrical and mechanical. Field potential-like (to monitor beat rate and QT/QTc surrogates), contractile force (pixel-based motion or pillar deflection), and perfusion (flow, oxygenation) signals. Where additional temporal resolution is needed in the assay, impedance and calcium imaging are supported.
What is the importance of microfluidics in this case? Can we not simply make use of organoids?
Morphology: Organoids are best; microfluidic chips are in control. You can tune up nutrient delivery, drug exposure, and endothelial-myocyte crosstalk with defined channel sizes, flow rates, and compartmentalization. It is that control that provides reproducible dose-response curves and reduces the assay variance.
Proximity of the mechanics to native myocardium.
The stiffness of the substrate can be set into the cardiac-relevant window (10-20 kPa), pacing is programmed (1-2 Hz habitual; faster frequencies during stress tests) and perfusion maintains lactate levels low. Coefficients of variation beat interval can be maintained to less than 1/10th over multi-day runs under not too much pressure control – sufficient to screen and longitudinal studies of patients.
What was the contribution of Microfluidics Innovation Center (MIC)?
Design and fabrication of microfluidic chips; flow engineering for long, bubble-free perfusion; automation of stimulus-response procedures; and transfer of the complete assay to a full-fledged prototype and SOP package. These are the purposes of MIC: to close the gap between discovery and deployment by fast-priming chips, non-failing tubing, and software to record each heartbeat.
What is the scalability of the platform of pharma-style screening?
CiStem architecture allows parallelization: multiplexed channels can be found on one chip, fluidic connectors have been standardized, and can be used with the plates. Practically, it is possible to use pacing, exchange of media automatically, and run panels (e.g., 8-32 conditions) in parallel, thus reducing cycle times to dose-response mapping.
What do you think this would be in a Horizon Europe consortium?
Precisely at the interface of translation. A heart-on-a-chip work package risk eliminates preclinical cardiology of therapeutic and advanced therapies, produces publishable physiology, and produces prototypes. Incorporated consortia, such as MIC, in microfluidic engineering, assay automation, and exploitation generally yield higher success rates than official success baselines; our consortia have seen a 2-fold increase in proposal hits when an applied microfluidics partner is in-house on the start day.
Are rare-disease or safety questions customizable on the chip?
Yes. A number of channel geometries, pacing regimes, ECM stiffness, endothelialization, and perfusion profiles can be customized. In safety pharmacology, you would prefer to see clean QT readouts and wash-in/wash-out kinetics; in rare disease, you would bias the co-culture and stimulation pattern to reveal genotype-specific phenotypes.
Reason to include MIC in your next cardiac/ multi-organ project?
In addition to designing devices, MIC has expertise in proposal writing, consortium coordination, and technology transfer. We co-develop prototypes with laboratories and companies regularly, and our involvement in European consortia has enabled groups to move more quickly from proof-of-concept to usability, microfluidics, automation, and data, all in harmony.