Microfluidic cell confiner for next-generation cell culture systems: MicroMechCell
Author
Christa Ivanova, PhD
Publication Date
September 14, 2017
Status
Keywords
Microfluidic cell confiner
Lab-on-a-chip
Autophagy
Mechanotransduction
High‑precision cell confinement
3D cell culture systems
Stem cell differentiation
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Cell culture systems do not reflect in vivo conditions. Although these systems are effective, they don’t consider the surrounding cells.
Cell confiner: introduction
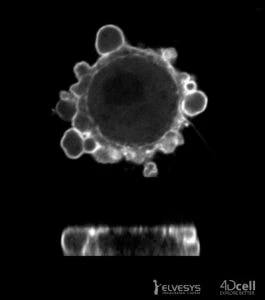
Cells are typically cultured on flat surfaces in petri dishes or flasks. Although these systems are very effective, they ignore the reality that cells in the body are surrounded by other cells and extracellular matrix and are constantly subjected to forces.
Specific cells experience additional types of strain: muscle, heart, and lung cells are under various types of repetitive strain, and the tumor environment is subjected to increased pressure. Our cell confiner should solve this problem.
A system that is easy to use for cell culture under confinement is not yet commercially available, and a market study demonstrates that it can be helpful for researchers or industrials. Therefore, creating an easy-to-use tool to confine cells with micrometer precision is a plus.
The cell confiner device allows confining of cells within two surfaces with micrometer precision. The space between the two surfaces is controlled by using micro polydimethylsiloxane (PDMS) pillars.
The micropillars are fabricated in the top confining surface, a glass slide. The glass slide is attached to a PDMS structure that acts as a piston. This piston is controlled with a vacuum pump, and the height of the confinement is thus controlled as well. The device can be used in a Petri dish, in a glass substrate, etc.
The device has a second version that allows confining cells in a multi-well plate. In this one, the vacuum pump is unnecessary since the pressure system is based on a modified lid containing PDMS blocks with confinement slides on the extremity.
Cell confiner: applications
To study cell dynamics triggered by /under mechanical effects:
- Migration
- Cell division
- Induced autophagy
- Mechanotransduction
- Mechanics of the nucleus
- Etc.
To co-culture cells
- Use with special substrates – functionalized, micro patterned, etc.
- Mimicking the natural cell environment
- Imaging
- Cell counting
The technology to confine the cells is already available and validated (Le Berre et al., Int. Bio. 2012). However, the MicroMechCell project aims to make the device viable for commercialization through the development of a more reliable system.
Explore the potential of the confiner with 4Dcell!
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 739692 (MICROMECHCELL project).
DISCLAIMER: The results reflect only the author’s point of view, and the EASME (Executive Agency for Small and Medium-sized Enterprises) is not responsible for any use that may be made of the information it contains.


Check our Projects
FAQ – Microfluidic cell confiner for next-generation cell culture systems: MicroMechCell
What was MicroMechCell about?
To make mechanobiology studyable under on-chip conditions that are reproducible, high-precision micromechanical devices are needed to apply uniform confinement to living cells – not too tight to control shape and stress, not too soft to crush them – to design such experimental conditions.
So what is so important about uniform confinement in cell culture?
Due to the sensitivity of gene expression, migration, and fate to small gradients of stress or strain. A lever that provides equal pressure across the entire cell population eliminates a conventional source of noise, making comparisons across laboratories much more justifiable. The project directly addressed the unreliability of previous pressure application methods.
What issue with the existing tools did the team address?
The design principle had met its goal, yet the pressure actuation was not entirely stable and could not be commercialized. MicroMechCell further re-engineered the actuation to enable controlled, reproducible force delivery and to incorporate it into a lab-on-a-chip.
What is the practical use of the leverage?
A microfluidic chip is used to culture cells; a lever provides a set confinement (i.e., a specified gap or force), and media, drugs, or cytokines are perfused. Under tightly controlled mechanics, readouts, morphology, nuclear shape, viability, and transcriptional reporters are then harvested. The difference is that mechanical inputs are an experimental variable, and not a nuisance.
Who financed it, and on what magnitude?
MicroMechCell was an action under Horizon 2020 (SME Instrument), with grant agreement No. 739692. Published budget amounts show that the overall potential cost was approximately EUR88,750, with an investment of 100 per cent in a small, focused development sprint rather than a massive consortium initiative.
What are the research problems that a uniform confinement tool opens?
Three large ones: (i) the influence of mechanical stress on differentiation (e.g., stem cells in compressive force), (ii) the control of mechanically primed phenotypes by immune cells in tight spaces, and (iii) the interaction between drugs and mechanically primed phenotypes. they are orthodox lines of mechanobiology and 3D culture.
What is the implication of this with regard to microfluidics and MEMS best practice?
Lever architecture complements MEMS/microfluidic stacks by enabling rapid thermal equilibration, tight geometries, sensor integration, and miniature reagent volumes. Recent MEMS recommendations emphasize biocompatibility, traceability of calibration, and standard mechanical readouts- precisely the level this type of device requires to be adopted.
What distinguishes this from soft, deformable PDMS channels used by people now?
The lever of MicroMechCell aims for a more uniform and controllable confinement, more like a mechanical dial than a by-product of flow, making such experiments easier to redo and compare.
I am developing a Horizon Europe consortium- where should MIC be placed outside this project?
MIC is a microfluidics SME specializing in regularly participating in EU consortia to provide hardware, automation, and measurement components for complex bioassays. We prepare offers together, model work packages based on prototype deliverables, and risk-proof manufacturable designs. Consortia incorporating MIC prototype-first model usually claim success rates that are twice the official baseline at similar calls- a trend we put down to obvious technical way, believable milestones, and early demonstrators.