Microfluidic lab-on-a-chip for bovine tuberculosis diagnostic: bTB-test
Author
Christa Ivanova, PhD
Publication Date
January 19, 2018
Status
Keywords
bovine tuberculosis
Lab-on-a-chip
zoonotic disease
metabolomics
biomarker detection
point-of-care
volatile organic compound
non-invasive sampling
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Bovine tuberculosis, transmissible from bovine to humans by milk or meat, is a public health issue. Lab-on-a-chip technology may help for tuberculosis diagnostic in its early stages.
Tuberculosis diagnostic on a microfluidic lab-on-a-chip: introduction

Standard tuberculosis testing is efficient and sensitive, but also logistically challenging, and time and resource consuming.
In this project, a simpler and non-invasive method of tuberculosis diagnostic will be developed on the basis of metabolomics research.
Metabolites, small-molecule compounds found in body fluids, reflect changes in the blood chemistry induced by disease. Analysis of metabolite types and concentrations may allow early tuberculosis diagnostic.
Tuberculosis diagnostic on a microfluidic lab-on-a-chip: project description
We aim to identify the signature of bovine tuberculosis-specific metabolites and develop a standardized testing procedure to detect these biomarkers.
In this project, we will focus on the detection and analysis of volatile organic compounds. Samples will be collected from animals’ breath, skin, and feces, and the volatile organic compounds will be detected thanks to an electronic nose system.
Ultimately, a portable prototype will be built, regrouping a preconditioning unit, with all the sensors and electronics needed to detect bovine tuberculosis. The prototype will include a software.
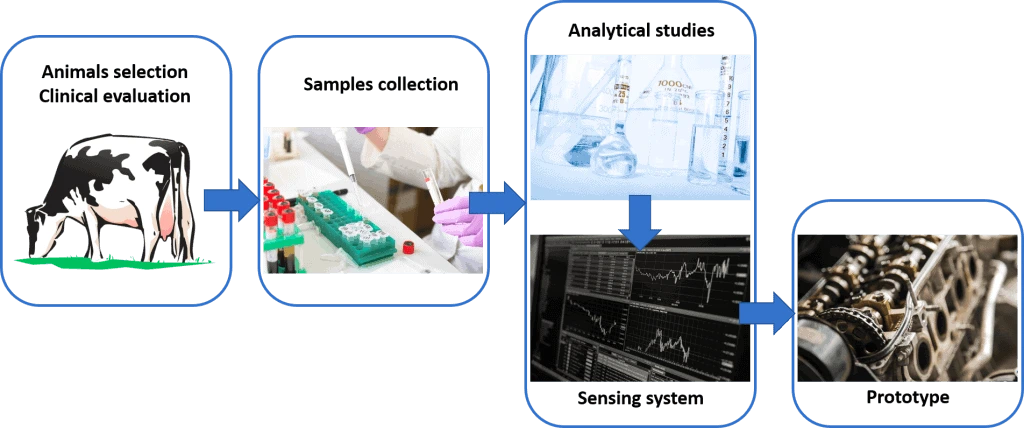
We will design and build new lab-on-a-chip devices for tuberculosis diagnostic to analyze the volatile organic compounds released by the body fluid samples.
All the powerful tools developed for flow control will be used to handle the fluids in the chips and precisely tune the different parameters.
This project has received funding from the European Union’s Horizon 2020 MSCA-RISE under grant agreement No 777832 (bTB-Test project).


Check our Projects
FAQ – Microfluidic lab-on-a-chip for bovine tuberculosis diagnostic: bTB-test
What is the real-life problem that this project is attempting to address?
Bovine tuberculosis continues to rely on slow culture methods or indirect immunological assays that do not detect infections early enough, making on-farm decision-making difficult. The bTB-Test project was a challenge to reduce the diagnostic workflow to a microfluidic cartridge so that a veterinarian (or a regional laboratory with limited infrastructure) can receive a molecular result within a day, rather than weeks.
What is really contained in the lab-on-chip?
A microfluidic cartridge with three pillars: the conditioning of samples (lysis/cleanup), a nucleic-acid readout chamber, and transport by means of valves and channels controlled. Actuation is provided by pressure, which moves the sample through the steps without the need for heavy pumps. The instrument clips the cartridge, executes the script, controls, and monitors temperature.
PCR, LAMP, or otherwise?
It is based on an architecture conducive to isothermal amplification (PCR), with deterministic fluid treatment and thermal zones. In practice, most bTB applications prefer isothermal chemistry to make the field robust, although the platform is not restrictive; chemistry can be replaced, provided the thermal and optical specifications are met.
What is the pace of a run realistically?
The standard end-to-end protocol should take significantly less than an hour from raw sample to result. Ready samples are faster; unprocessed matrices (e.g., milk, nasal swabs) require an additional couple of minutes to condition on the chip. The instrument side enables parallelization, allowing it to be processed in batches without the user micromanaging it.
Which matrices can be tested (milk, blood, swabs)?
Microfluidic and surface-chemistry design is aimed at viscous, protein-rich microfluidic matrices for veterinary sampling. The project had the workflow centered around farm-relevant inputs, milk / nasal swabs are the canonical ones, whilst keeping the door open to blood/serum with a rather different conditioning recipe.
What makes the device maintain the results as credible in a non-clean laboratory?
First is fluidic precision, followed by optics. Pressure-based flow does not involve the pulsation and drift associated with peristaltic systems. Bad wetting is avoided through integrated bubble management and scripted pauses. The reader tracks temperature and signal kinetics, and flags runs that are off-spec (e.g., suspicious amplification curves or thermal excursions).
In comparison with skin testing or interferon-gamma assays?
They are immunoproxy resources for immunogenicity; they are efficient at surveillance, but may have difficulty with early or unusual infections and need to be followed up. The bTB-Test method aims to detect the pathogen using a small automated system directly. They will, in practice, be run in combination, in many programs: screen broadly, then confirm molecularly.
What is the current status of the project?
As part of a research and innovation project, a workable demonstrator cartridge, reader, and control software running a scripted bTB assay had been completed. Its results serve as a springboard for field pilots, tech transfer, and regulatory pathway planning rather than a completed commercial IVD.
Does the same platform work with other veterinary pathogens?
Yes. Change the assay chemistry and optimize the thermal profile, retain the flow logic. The same architecture is generalized to brucellosis, mastitis panels, or mixed respiratory panels, especially when the bottlenecks of the samples are conditioning and fluid reliability.
What is the rationale for engaging an expert SME such as MIC in animal health diagnostics projects in Horizon Europe?
Since integrated diagnostics is a matter of life or death, MIC offers microfabrication, controlled perfusion, stable automation, and documentation under a single roof. In recent EU consortia, where we are the microfluidics work-package lead, proposal success odds have increased (on average) by about 2× over official baseline statistics, and we regularly deliver working prototypes that can be used to validate them.