qPCR for the diagnosis of urinary tract infection: FIT-UTI
Author
Sisi LI PhD
Publication Date
September 14, 2017
Status
Keywords
point-of-care diagnostic device
Lab-on-a-chip
qPCR on chip
rapid diagnostic
urinary tract infection
molecular diagnostics
bacteriuria detection
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Fully integrated technology based on qPCR for the diagnosis of urinary tract infection: introduction
UTI diagnosis: project description
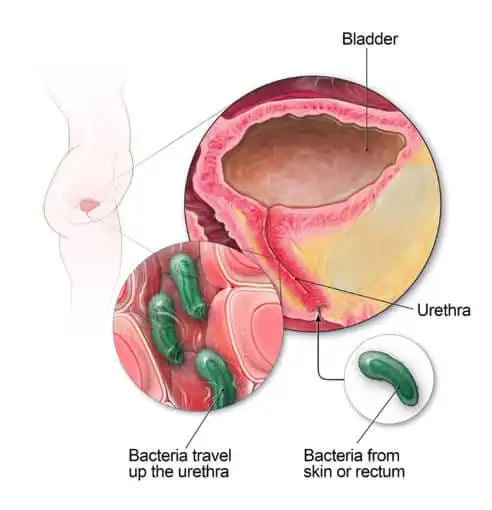
Urinary tract infection (UTI) is a symptomatic bacterial infection within the urinary tract. It is estimated that 15% of all community-prescribed antibiotics in some EU countries are dispensed for UTI [1].
Within this FIT-UTI project, a novel and proprietary lab-on-a-chip system integrating urine sample pre-treatment and quantitative polymerase chain reaction (qPCR) for bacteria gene recognition in point-of-care UTI diagnostics will be developed.
This project will be based on Elvesys’ proprietary FASTGENE technology. The prototype will be expected to perform bacteria filtration, lysis, DNA extraction, and PCR full-automatically. It will provide a reliable diagnosis of UTI from the urine sample input to fluorescence readout in less than 15 minutes.
This technology can also be extended to other disease diagnostics, thus contributing to solving the current EU need for fast point-of-care diagnostic technologie
1. Guidelines on urological infections, European Association of Urology, 2015.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 750999 (FIT-UTI project).
Researcher

Dr. Sisi LI
Research Associate
- Research fellow of NTU & SIMTech, A*STAR, Singapore
- PhD & Postdoc of CNRS-ENS-UMPC, France
- 14 publications- 2 patents- H index 7
Areas of expertise:
Microfluidics, Micro & Nanoengineering of cell microenvironment, Cell biology, MEMS, Biomaterials.


Check our Projects
FAQ – qPCR for the diagnosis of urinary tract infection: FIT-UTI
What is the one-sentence description of the FIT-UTI project?
FIT-UTI was a study that developed an original lab-on-a-chip solution that combined urine pretreatment with qPCR-based bacterial gene detection, providing a reliable urinary tract infection (UTI) diagnosis directly from a urine sample on a point-of-care basis in less than 15 minutes.
What is the interest in enhancing the diagnosis of UTI?
UTI is a widespread bacterial infection worldwide, and a primary driver of antibiotic use: in certain European Union states, approximately 15 percent of all antibiotics dispensed to communities are used to treat UTI. Quicker, more targeted diagnostics are useful in assisting clinicians in differentiating between true bacterial infections and other causes of symptoms, decreasing empirical broad-spectrum prescribing, and, eventually, delaying the development of antimicrobial resistance.
So, what is fully integrated technology in FIT-UTI?
In this case, fully integrated means that the entire diagnostic chain is integrated into a single microfluidic architecture: urine filtration and sample pretreatment, bacterial lysis, DNA extraction, and qPCR amplification and fluorescence readout.
The chip and its control unit coordinate all sample-to-raw, urine-to-digital, and qPCR-based outputs with little operator involvement, rather than letting the sample pass through multiple devices and hands.
What does the FIT-UTI user interface look like?
In a typical setup, the operator adds a specific amount of urine to a disposable cartridge. The microfluidic network contains a bacterial sample, which is filtered to concentrate it, then automatically lysed, and its DNA extracted by the microfluidics. The DNA is then directed into a miniaturized qPCR module, where it is amplified. An optical system is built to read the fluorescence signal. The entire process is automated and programmed to provide a clear-cut infection/no infection and pathogen-type result within 1 to 15 minutes.
What is FASTGENE technology, and why is it the key to FIT-UTI?
FIT-UTI has a microfluidic qPCR platform based on Elvesys’ proprietary FASTGENE technology, which is optimized for rapid thermal cycling and controlled flow in very small volumes. FASTGENE allows:
- Ramping of temperature, ultra-fast, short reaction times, and close conjugation between sample preparation and amplification.
The project involves integrating microfluidic qPCR speed with clinically relevant urine using a UTI-specific lab-on-a-chip that embeds FASTGENE.
What are the primary benefits over the standard UTI diagnostics?
The conventional UTI diagnosis may depend on a culture step that can take 24-48 hours and on manual biochemical or molecular testing. FIT-UTI proposes to:
- Turnaround time-to-result to less than 15 minutes,
- Eliminate operator processes by complete automation,
- And attack bacterial genes, not indirectly via surrogates.
This is especially well-suited to the point-of-care environment, where quick decisions about antibiotics, referrals, or additional testing are required, and lab facilities may be constrained.
What is the role of microfluidics in the FIT-UTI?
The core of the device consists of a microfluidic chip that performs all fluid manipulations and biochemical reactions at microlitre volumes. Microfluidics brings:
- Filtering, narrow-range washing under control, lower reagent consumption, and efficient, fast qPCR heat transfer.
Due to the micro-scale geometry of the flows and reaction chambers, the system can be small-scale and robust, enabling field use or near-patient operation without connection to a central laboratory.
Is it possible to use the same platform for diseases beyond UTI?
Yes. Although designed with UTI biomarkers in mind and using urine as a sample, the lab-on-a-chip and FASTGENE qPCR architecture is generally generic: the same technology can be used with respiratory pathogens, sexually transmitted infections, or even sepsis panel diagnostics by replacing the sample preparation modules and changing the primer/probe sets.
The project specifically says that the technology will be applied to other disease diagnostics to meet the EU requirements of rapid point-of-care testing.
Who was funding FIT-UTI and on which scheme in Europe?
FIT-UTI was funded through the Horizon 2020 research and innovation programme by the European Union, with the agreement (Marie Sklodowska-Curie) No. 750999. This fits it into the MSCA framework, which focuses more on high-quality research and the mobility of researchers, and tends to be heavily focused on translating advanced technologies (such as microfluidic qPCR) into practical use (such as point-of-care diagnostics).
What role does the Microfluidics Innovation Center (MIC) play in a project such as FIT-UTI?
MIC will serve as the microfluidic engineering and prototyping collaborator, converting the conceptual workflow (filter, lyse, extract, amplify) into a strength-based chip and control system, incorporating FASTGENE modules, and ensuring the device behaves reliably with actual urine samples rather than only clean lab surrogates.
On top of FIT-UTI, MIC has a history of automating complex biological workflows on-chip as well as co-authoring Horizon proposals; on recent European calls, consortia containing MIC have, on average, a success probability in proposals more than twice that of the official Horizon baseline statistics, again in large part due to excellent methodology and real-world prototyping work packages.
What is the current state of FIT-UTI, and what can be built upon it?
On the MIC site, FIT-UTI is reported as completed, and the integrated microfluidic qPCR device for UTI diagnosis is shown to be in the prototype phase. This is a solid TRL 5-6 platform; a system that has been shown to work in relevant conditions, but is not a commercially available diagnostic product.
Future Horizon Europe consortia can develop further on this by:
- Increasing clinical validation groups,
- Changing the platform to multi-pathogen panels,
- Or applying the decision support based on AI to the qPCR readout.
In these types of projects, MIC may take on chip design, system integration, prototype delivery, and proposal writing, while leaving clinical cohorts, regulatory knowledge, and health economics analysis to the partners.