Study of liver sinusoids’ fenestrations with microfluidics: DeLIVER
Author
Alessandra Dellaquila, PhD
Publication Date
January 28, 2018
Status
Keywords
liver sinusoids fenestrations
Liver Sinusoid‑on‑chip
liver sinusoidal endothelial cells
nanoscale imaging
quantitative phase imaging
endothelial transport
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Microfluidics device to study the fenestrations of liver sinusoids: introduction
If hepatocytes have been intensely studied, the role of non-parenchymal cells and their interaction with hepatocytes are still not well documented.
Specifically, the endothelial cells of liver sinusoids are responsible for blood clearance, and the endothelial transport is ensured by nanosized pores called fenestrations.
Their small size (50-200 nm) and dynamic structures hinder their characterization in living cells using currently available microscopy techniques.
Organ-on-a-chip to study fenestrations in endothelial cells of liver sinudoids: project description
Thus, It is desirable to design functionally engineered in vitro models to profoundly investigate the function and dynamics of the fenestrations of liver sinusoids to understand liver pathophysiology fully.
This project will address this issue by developing a novel high-speed super-resolution microscope capable of imaging the evolution of fenestrations over time and space.
This innovative microscopy technique uses periodic interference light patterns to pass the diffraction limit of light, thus imaging nanometric structures.
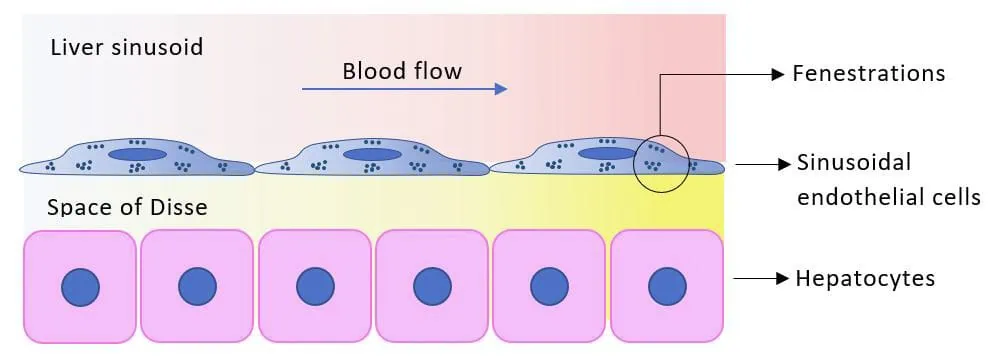
Based on its expertise in organ-on-chip devices and flow control systems, we will develop a new chip design for 3D microfluidic cultivation and cell manipulation, meeting the requirements of this new microscope.
This organ-on-a-chip will provide a powerful tool to study the fenestrations’ response to several stimuli (drugs, molecules, metabolites, rapidly changing flow conditions…) and highlight the differences between healthy and unhealthy liver sinusoidal endothelial cells.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 766181 (DeLIVER project).
Researcher

Alessandra Dellaquila
PhD Candidate
- Research associate (Institute of Science and Technology for Ceramics, Italy)
- Master of Science in Biomedical Engineering (Politecnico di Torino, Italy)
Areas of expertise:
Biomedical engineering, biomechanics, data analysis, microfluidics.
Do you need more information? You can have a look at Alessandra’s review about optical detection in microfluidics.


Check our Projects
FAQ – Study of liver sinusoids' fenestrations with microfluidics: DeLIVER
What is the scientific gap that DeLIVER fills?
We are familiar with hepatocytes; the non-parenchymal compartment, namely liver sinusoidal endothelial cells (LSECs), is less mapped out. LSECs control blood clearance by nanoscale fenestrations (≈50-200 nm), which, despite being small, dynamic, and difficult to visualize live, are remarkably difficult to visualize in living organisms. DeLIVER then embarked on rendering those structures to be apparent and measurable under realistic flow.
What did the project construct?
Two things concurrently:
(i) a fast super-resolution microscope operating on periodic patterns of interference, which breaks the diffraction limit (a SIM-like strategy), and
(ii) a new organ-on-chip that is differentiable to 3D microfluidics culture and cell manipulation that is not limited by optical and mechanical factors to the same microscope. When combined, the combination allows tracking dynamic fenestration across space and time.
Why not simply make use of a standard confocal microscope?
Classic confocal is not guaranteed to sharpen pores smaller than 200nm in fast living and moving specimens, and long dwell time blurs the biology you are investigating. Patterned-illumination super-resolution modality images the nanometric structures at a faster rate, whereas the chip maintains the cells in a realistic perfused condition, thus the morphology, transport, and signaling are preserved.
What are some of the questions I can answer using this platform?
Yes. The microscope had a high-speed super-resolution design and the chip enables quick switching of the conditions (drugs, media, flow rates). The combination of that pairing facilitates time-resolved imaging of the opening/closing cycles of a fenestration as opposed to individual snapshots.
How alive is it, can it record high change rates?
MIC designs the fluidic system, which feeds the cells under control, imposes controlled flow and shear forces, and injects well-characterized nanoparticle formulations. Consider it the chip’s circulation system: strong perfusion, accurate dosing, and controls that make experiments reproducible. MIC also assists in setting the test conditions and throughput with the consortium.
Sample prep and cell sources, what about them?
The organ-on-chip is based on liver sinusoidal endothelial cells (primary or model lines) in a 3D-compatible layout. Since fenestrations are both mechanically and chemically sensitive, the protocol focuses on stable perfusion, clean surface chemistry, and the delivery of a stimulus with short latency, which are programmed into the flow-control logic.
Specifically, what did MIC (Microfluidics Innovation Center) do?
MIC was the first to integrate organ-on-chip engineering with flow control, developing a chip that addresses the optical constraints of the super-resolution arrangement and enables programming perfusion and stimuli. In practice: reproducible shear profiles, rapid media changing, on-chip manipulation to preserve LSECs in a healthy state, and to measure them.
Is this able to be combined with the screening of the drug or disease modeling?
That’s the point. Drug pulses, metabolites, or quickly changing flow can be compared between healthy and pathological LSECs using the platform. The fact that the readout is a quantitative time-resolved readout means that you can generate dose-response curves of fenestration density/size and connect them to functional transport endpoints.
We are forming a Horizon Europe consortium, what can MIC do to improve our chances?
In addition to chip fabrication, MIC also handles automation, microfabrication, and valorization, and assists with organizing the proposal (work plan, risks, data interoperability). In recent bids, one-out-of-six to one-out-of-eight consortia with special SMEs, such as MIC, have achieved about twice the success rate as official baselines, mostly on the Implementation/Impact scores, and have prototype-quality hardware at the start of the project.