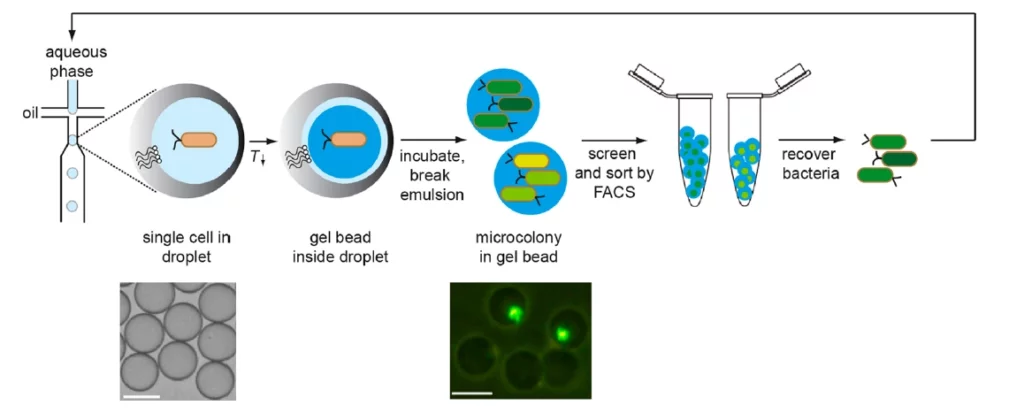
Overview of the screening method for microcolonies by Duarte, J. et al.
MICROCOLONIES SORTING FOR FACS
Avoid double emulsions (water-in-oil-in water)
For FACS-based sorting
Analyze a large number of cells in a short amount of time
Microcolonies sorting for FACS
Fluorescence activated cell sorting (FACS) has a high level of flexibility, the ability to simultaneously screen multiple parameters, and an unmatched throughput of >107 events/hour. It sorts individual cells according to their fluorescence.
However, in synthetic biology, FACS has not yet been widely used for screening libraries of synthetic circuits [1]. That’s why, microcolonies sorting make it possible to take advantage of the FACS technique without being reliant on single cell readouts. The microcolonies can be confined in hydrogel beads [2-4].
Whether you want to do microcolonies sorting or single cell screening, we offer you an all-in-one setup to prepare your sample for FACS.
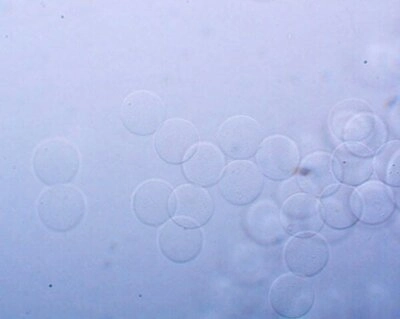
The pack allows you to encapsulate cells in alginate beads.
Microcolonies in alginate beads for FACS sorting setup
An all-in-one pack guarantees a good compatibility between the different instruments, allows you to start your experiment right away, is piloted by a single software and can be used for other different applications. These are a few arguments why a pack is the easiest way to setup a microfluidic experiment.
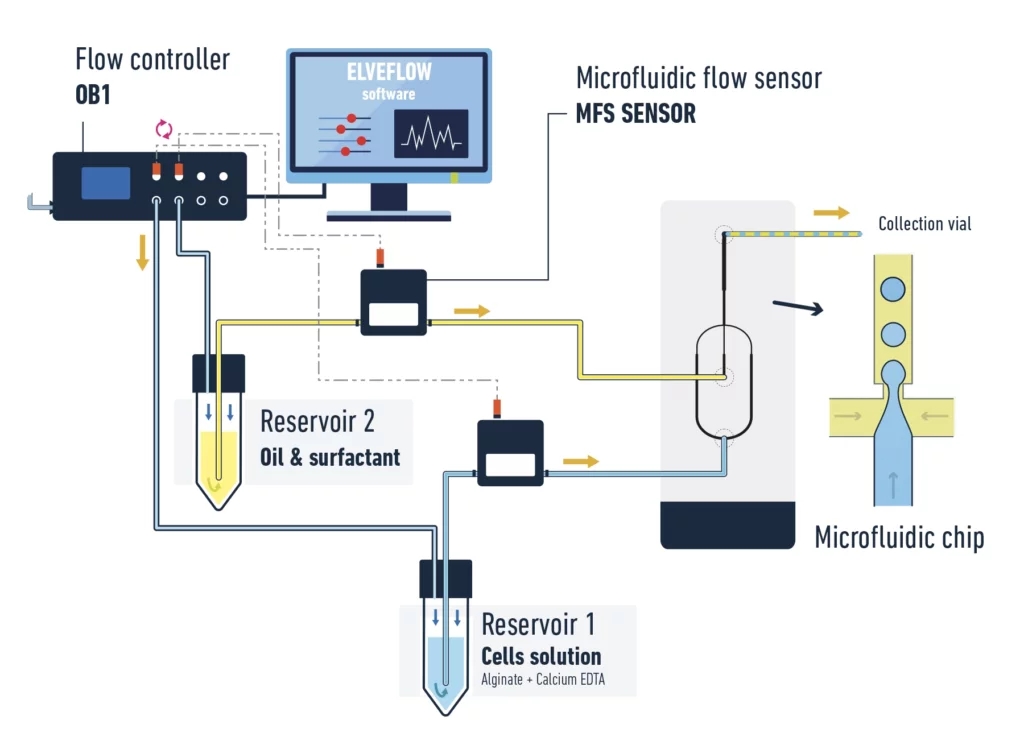
A typical pack contains:
- OB1 flow controller (Elveflow) with at least two channels 0/2000 mbar
- 2x flow sensors MFS 0/7 µL/min (Elveflow)
- Kit starter pack luer lock with fittings and tubings
- 2x 15 mL Falcon reservoirs
- Microfluidic chip (hydrophobic channels)
- Microscope for observation (optional)
- Fast camera to register droplets (optional)
- ESI control software (automated sequences possible) (Elveflow)
Our setup allows you to entrap cells in alginate beads. Once incubated, they grow into microcolonies for fluorescence activated cell sorting (figure 1).
You can also use the setup for single cell screening as well.
The chip used in the setup
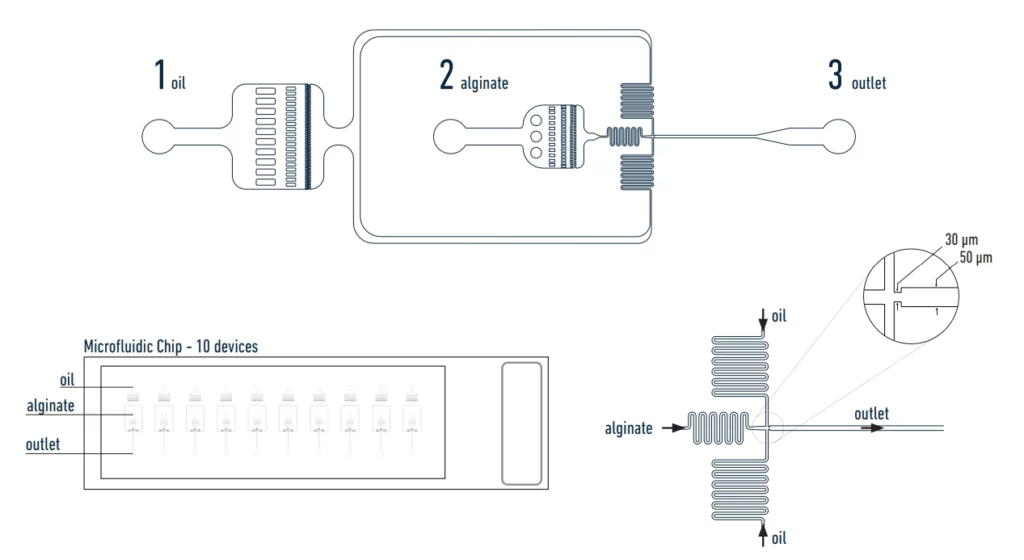
The used chemicals for sample preparation:
- Alginate (low viscosity)
- Calcium-EDTA – Merck, SKU: ED2SC-100G
- Acetic acid – Merck, SKU: 33209-1L-M
- HFE7500 oil with 2% surfactant
- Droplet breaking solution
- 1 vial of droplet oil
References
1. Davies, D. (2012) Cell separations by flow cytometry. Methods Mol. Biol. 878, 185−199.
2. Weaver, J. C., Bliss, J. G., Powell, K. T., Harrison, G. I., and Williams, G. B. (1991) Rapid clonal growth measurements at the single-cell level: gel microdroplets and flow cytometry. Nat. Biotechnol. 9, 873−877.
3. Sahar, E., Nir, R., and Lamed, R. (1994) Flow cytometric analysis of entire microbial colonies. Cytometry 15, 213−221.
4. Zengler, K., Toledo, G., Rappe, M., Elkins, J., Mathur, E. J., Short, J. M., and Keller, M. (2002) Cultivating the uncultured. Proc. Natl. Acad. Sci. U. S. A. 99, 15681−15686.
5. Duarte, J. M., Barbier, I., & Schaerli, Y. (2017). Bacterial microcolonies in gel beads for high-throughput screening of libraries in synthetic biology. ACS synthetic biology, 6(11), 1988-1995.
Microfluidic FACS applications
Microfluidic fluorescence-activated cell sorting (FACS) is a powerful tool used to isolate and sort specific cells from a heterogeneous population of cells.
It has a wide range of applications in various fields, including biology, medicine, and biotechnology.
Some applications include:
- Cell analysis: Microfluidic FACS can be used to analyze different types of cells, such as stem cells, cancer cells, and immune cells, by detecting specific markers or proteins expressed on their surface.
- Single-cell analysis: Microfluidic FACS allows for the isolation and analysis of individual cells, which is particularly useful in studying cell heterogeneity and identifying rare cell populations.
- Cell-based assays: Microfluidic FACS can be used to sort cells based on their response to specific stimuli, such as drugs or toxins, enabling the development of high-throughput cell-based assays.
- Cell therapy: Microfluidic FACS can be used to isolate and enrich specific cell types, such as stem cells or immune cells, for use in cell therapy applications.
- Microbial analysis: Microfluidic FACS can be used to sort microbial cells based on their characteristics, such as size, shape, and fluorescence, enabling the study of microbial communities and their interactions.
Overall, microfluidic FACS provides a powerful tool for cell analysis, sorting, and manipulation, which has a wide range of applications in various fields.
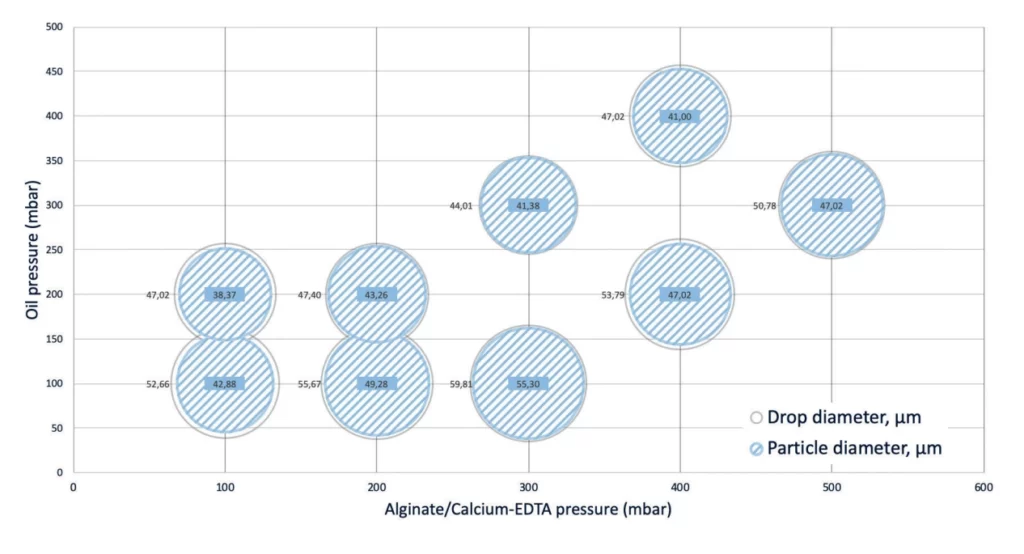
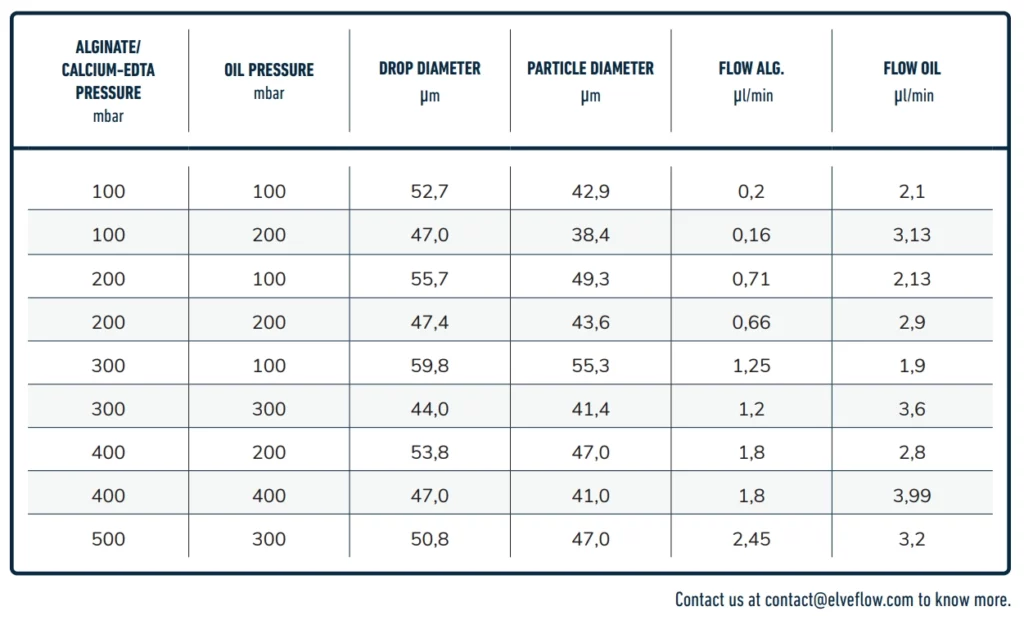
Droplets' size and pressure
The size and frequency of the droplets will depend on the pressure, flow rate and viscosity of the liquids used to generate the alginate beads. See droplet size/pressure diagram below.