Stage Top Incubator
Culture cells on top of the microscope stage for long periods
Accurate temperature control
Keep your cells at 37°C ± 0.5°C outside the CO2 incubator
High quality image gathering
Bottom glass window can be adjusted to your microscopy needs
Up to 3 simultaneous cell cultures
Test different conditions in the same system

Need a microfluidic SME partner for your Horizon Europe project?
Stage top incubator setup
The stage top incubator is compact and can be easily manipulated to be put on a microscope while maintaining dynamic perfusion and temperature control. The stage-leading incubator can hold up to 3 chips simultaneously and can easily be stacked for parallelization.

A stage top incubator pack contains:
Flow sensor (Galileo, MIC)
Software (Galileo user interface)
Stage top incubator
Flow controller
Several Eppendorf or Falcon reservoirs
Tubings and luers
Microfluidic chips, if needed
User guide
The flow controller performs the media flow for dynamic perfusion, which applies pressure to the reservoirs to induce the flow. A flow sensor is then added to the setup with a feedback loop to the flow controller that allows maintenance of reasonable control of the flow rate inside the stage top incubator during the experiment.
This pack can be combined with microfluidic functions like recirculation, sequential injection, bubble trapping, cell-cell interaction monitoring, etc.
Our Research & Innovation team is exploring this product. Contact our experts if you are interested in it, its applications, or possible variations to better suit your needs!
Static live cell imaging: Microglial cell culture
According to the protocol published by Sepulveda et al., Glia, 2016, primary microglial cells were isolated from mice pups’ brains.
Primary mouse microglial cells were prepared and kindly provided by Patrick P. Michel and Rocio Gimenez from the Brain and Spine Institute in the frame of the LOCAI project.
Cells were passaged after one week of isolation onto Ibidi chips (µ-Slide III 3D Perfusion, cat. 80376) at 150 000 cells/mL density. Cell medium was supplemented with FBS and PS. HEPES in powder was added to a final concentration of 20 mM. Once the HEPES dissolved, the pH was corrected and brought to 7.2 using Na 1 N, and then the media was filtered using a 0,22 filter. Cells were left to attach overnight in an incubator at 37°C and 5% CO2.
Hardware
Flow sensor (Galileo, MIC)
Flow controller
Tubings (1/32″ ID), fittings and reservoirs
IBIDI µ-Slide III 3D perfusion
8 cm of 100 µm inner diameter microfluidic resistance
Microfluidic male and female Luer to ¼” -28 connectors
Laminar flow hood
Standard Incubator for cell attachment, stage top Incubator for experiment
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM)
Penicillin/Streptomycin, 10,000 U/ml, 10mg/ml Streptomycin, PAN biotech
FBS, 0.2 µm sterile filtered, PAN Biotech
HEPES is used to buffer pH in the absence of CO2 control.
Software
Flow sensor (Galileo, MIC)
Thermal chamber software
Design of the chip
| Components | Specifications |
|---|---|
| Interface type | Female Luer |
| Well diameter | 5.5 mm |
| Well height | 1.7 mm |
| Volume per well | 30 μl |
| Material | Lbidi Polymer Coverslip |
| Surface treatment | Bitrate |
| Growth area per well | 25 mm2 |
As a proof-of-concept of the efficacy of the stage top incubator in maintaining ideal conditions for live cell imaging, primary mouse microglial cells were cultured in static conditions for 48h inside it. The figure below show the results after 8h and 48h of stimulation with LPS.
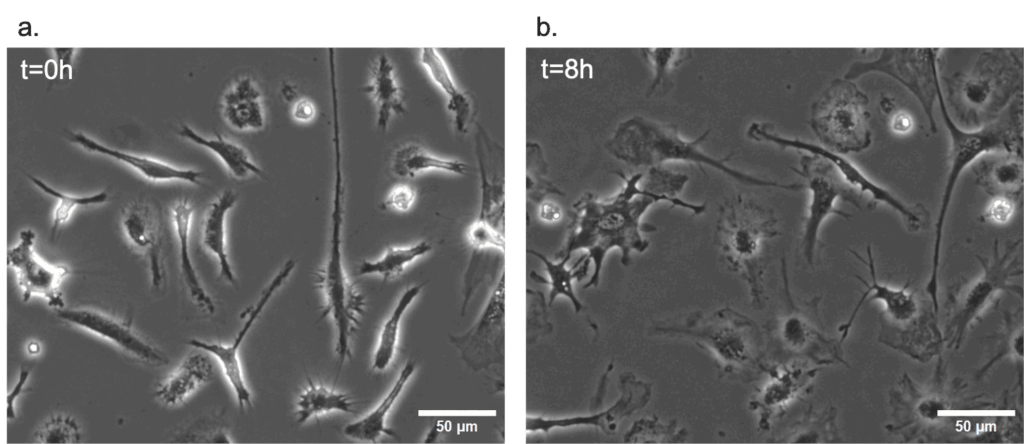
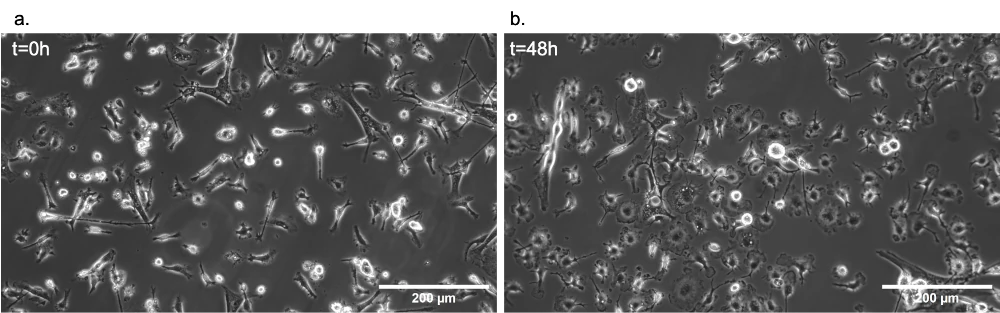
Dynamic live cell imaging: HEK293 cells under flow
Downscaling cell cultures to the organ-on-chip technology implies that heat and mass transfer much faster and more efficiently in or out of the chip. This allows the media to reach the right temperature quickly after entering the Stage Top Incubator, even at high flow rates.
HEK293 cells were maintained in standard cell biology practices, with passages twice weekly.
Hardware
Flow sensor (Galileo, MIC)
Flow controller
Tubings (1/32″ ID), fittings and reservoirs
IBIDI µ-Slide Luer – 400 µm in height (permeable, polymer coverslip, and impermeable, glass coverslip
10 cm of 175 µm inner diameter microfluidic resistance
Microfluidic male and female Luer to ¼”-28 connectors
Laminar flow hood
Standard Incubator for cell attachment, stage top Incubator for experiment
pH optical sensor, in-house developed flow cell
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM)
Penicillin/Streptomycin, 10,000 U/ml, 10mg/ml Streptomycin, PAN biotech
Software
Galileo user interface
Thermal chamber software
Design of the chip
Gas impermeable – Glass Coverslip
| Components | Specifications |
|---|---|
| Outer dimensions (w x l) | 25.5 x 75.5 mm² |
| Channel length | 50 mm |
| Channel width | 5 mm |
| Adapters | Female Luer |
| Volume per reservoir | 60 μl |
| Growth area | 2.5 cm2 |
| Bottom: Glass coverslip No. 1.5H, selected quality, 170 µm +/- 5 µm |
Gas permeable – Polymer Coverslip
| Components | Specifications |
|---|---|
| Outer dimensions (w x l) | 25.5 x 75.5 mm² |
| Channel length | 50 mm |
| Channel width | 5 mm |
| Adapters | Female Luer |
| Volume per reservoir | 60 μl |
| Growth area | 2.5 cm2 |
| Coating area | 5.2 / 5.4 / 5.6 / 5.8 cm2 |
| Bottom: IBIDI polymer coverslip |
The stage top incubator was used to culture HEK293 cells under flow on the microscope stage for 60 hours. The pH was continuously monitored, and images were taken at defined time points. The gas permeability of the chips was assessed to understand the influence of lack of CO2 control on cell culture outside the CO2 incubator. Cells grew and multiplied in both conditions, with the impenetrable chip showing more considerable pH variations without negatively impacting cell doubling.
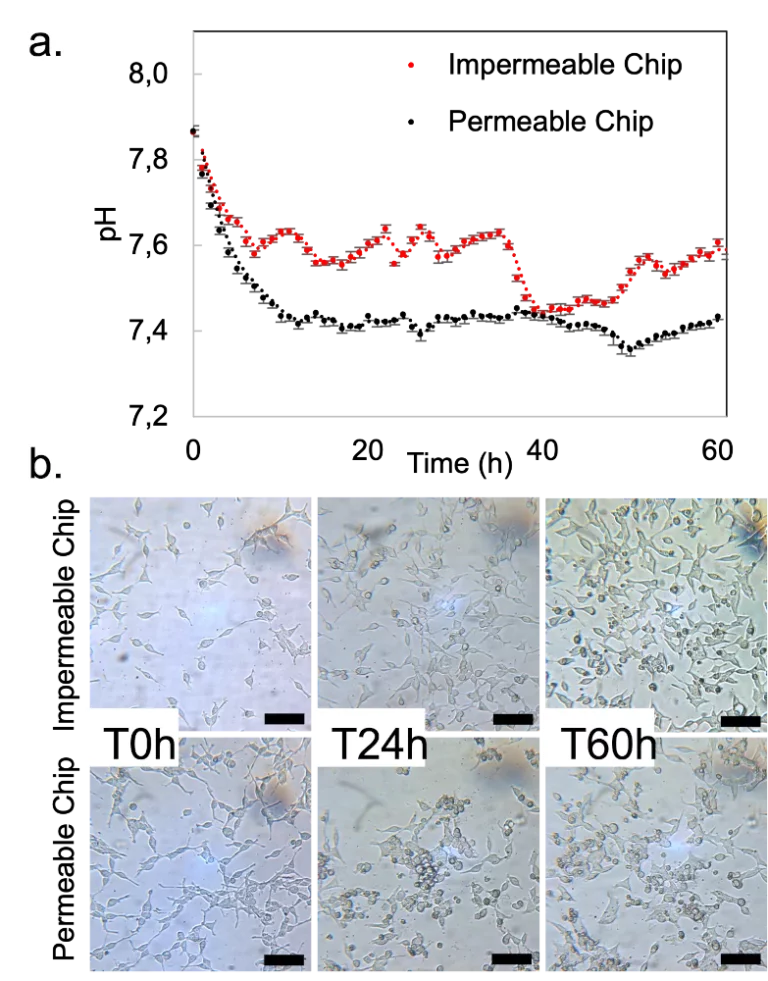
Stage top incubator specifications
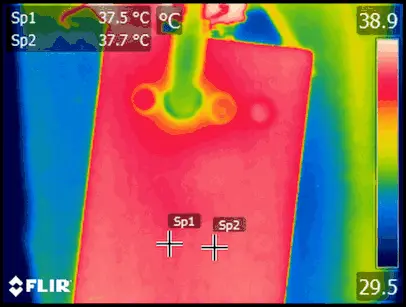
The Stage Top Incubator was designed to allow cell culture on top of the microscope stage for live cell imaging. As the animation shows, it will enable homogenous temperature transfer across its working area.
The solutions are thermalized as they enter the chip, keeping a homogenous temperature throughout. Thus, solutions can be preheated at the desired temperature before entering the chip.
The table below summarizes its main specifications.
| Characteristics | Specifications |
|---|---|
| Dimensions (mm) | 30.5 x 130 x 168 (h x w x l) |
| Base K- Frame | 3.5 x 110 x 160 (h x w x l) |
| Dimensions of internal usable space | 25 X 89 x 130 (h x w x l) |
| Dimensions of the bottom glass (ITO glass) | 1) 72 X 110 with a thickness of 1.1 mm 2) 50 x 25 with a thickness of 0.6mm 3) 50 x 22 with a thickness of 0.12 mm |
| Temperature range | Room temperature to 70 oC |
| Temperature accuracy | ± 0,5 oC |
| External material | Aluminum and ITO glass |
The stage top incubator is controlled by a converter connected to software (both included). Also, it is compatible with 1/16″ and 1/32″ ID tubing.

Customize your stage top incubator
Several commercialized and laboratory-made microfluidic chips have been developed and tested recently to perform dynamic cell cultures efficiently. We can include one of them with different surface modifications inside the pack. A chip can also have several separate channels.
Our products and packs are fully customizable to fit your needs perfectly. Our microfluidic specialists and researchers will help you choose the best instruments and accessories and accompany you during the setup of the microfluidic platform until you can obtain your first experimental results.
Contact our experts to answer any questions about this stage top incubator for microfluidics pack and how it can match your specifications!
Frequently asked questions
Can the stage top incubator be sterilized?
Yes, we have developed a simple protocol for sterilization and cleaning that is provided along with the user guide.
Can the stage top incubator be placed inside the CO2 incubator?
The Stage Top Incubator was designed to be kept outside the CO2 incubator, but it can be adapted on demand.
Is the stage top incubator gas-tight?
No, the Stage Top Incubator allows gas exchange with the atmosphere.
Do the reservoirs of media need to be kept at 37°C?
No, the reservoirs can be kept at room temperature. The Stage Top Incubator was designed to ensure that the media reaches the cells at the desired temperature regardless of the temperature of the reservoirs.







Funding and Support
The Protomet, ACDC, and LOCAI projects results helped develop this instrument pack, with funding from
the European Union’s Horizon 2020 MSCA-ITN under grant agreement No 813873 (ProtoMet),
the European Union’s Horizon 2020 research and innovation program under grant agreement No 824060 (ACDC project),
and French National Research Agency (ANR) and the German Federal Ministry of Education and Research (BMBF) in the frame of the 1st German-French Joint Call for proposals on Artificial Intelligence.

