Microfluidic system for stem cell differentation: Pillarcell
Author
Christa Ivanova, PhD
Publication Date
December 12, 2013
Status
Keywords
tissue engineering
Regenerative medicine
pillar geometry
stem cell differentiation
Cell adhesion
Substrate stiffness control
micropatterned substrates
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
The Pillarcell research project aims to demonstrate the advantages of using microfluidic technologies to control stem cell migration and differentiation finely.
Microfluidics for stem cell differentiation: introduction
Stem cell differentiation control will be a crucial technology for future medical treatment and to fight human aging since stem cells have the inherent particularity to self-renew along the life cycle.
“Nature does nothing uselessly” (Aristotle: I.1253a8).
This is also true for the human body, a vastly complex organism comprising highly synchronized sub-systems and many individual cells. When body tissues or cells are placed in a dish, a flask, or a multi-well plate, they undergo substantial changes in the cellular microenvironment.
Their extracellular matrix becomes much more straightforward, soluble factors and nutrients usually present in their environment are provided differently, and cell-to-cell contact is modified. Such changes significantly affect cell-based assays, tissue engineering, and regenerative medicine performance.
Although much research has been done to improve in-vitro culture conditions, there is still no satisfactory technology that correctly mimics real in-vivo cellular microenvironments, mainly that induces stem cell migration and differentiation.
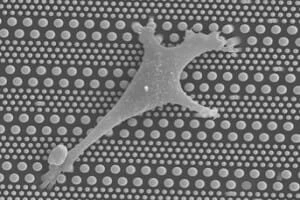
One reason is the lack of general platforms that allow for the high-precision regulation of cellular microenvironments. Microengineering techniques are now widely used to fabricate surface-textured culture substrates and synthetic extracellular matrix on the one hand and microfluidic devices for the dynamic control of soluble factors on the other.
Developing universal tools for stem cell differentiation: project description
In this project, we are systematically investigating stem cell migration and differentiation on patterned micro and nano-pillar arrays, with or without integration into microfluidic devices. By changing the geometrical parameters of the pillars, we mainly focus on the role of substrate stiffness on stem cell differentiation and migration.
We are working on this research project as part of a multidisciplinary consortium comprised of three academic research teams (one from the ENS specialized in nanofabrication technologies for cell biology, one from the Curie Institute specialized in cell polarity and migration on patterned surfaces, and another from the University of Paris-Diderot (Institut Jacques Monod) specialized in the biophysics of cell-based assays).
Our system can also be helpful for other cell-based assays, including adhesion, proliferation, differentiation, and apoptosis. Since the proposed fabrication technology can be used for large-scale manufacturing, it will be easy to convert our prototypes into industrial products for stem cell differentiation. To this end, we anticipate several clinical applications of such products, including tissue engineering and wound healing.
This project was funded by the French National Research Agency (Agence Nationale de la Recherche, ANR), under the No ANR13-NANO-0011 (PillarCell).

Check our Projects
FAQ – Microfluidic system for stem cell differentation: Pillarcell
What is PillarCell?
A cell culture platform based on microengineered arrays of micro- and nano-pillars, optionally coupled to microfluidic perfusion, to direct stem cell migration and differentiation. The pillars will provide you with a mechanical and topographical dial, and the channels will control soluble cues.
So, what is the solution to this in stem cell labs?
Reproducibility. When two labs use the same growth factor, the results are unlikely to match because the physical environments differ. More uniform pillar arrays and controlled perfusion eliminate a large portion of latent variability, thereby making differentiation protocols more transferable and defensible to reviewers.
Which sort of figures shall I make my plans around?
In the case of patterned substrates, common parameter sweeps are pillar diameters of 200nm-10mm, pitch 0.5-20mm, and aspect ratios of 5:1. In perfused forms, constant flows of 5-200 mLh-1 per channel are used to sustain nutrient/oxygen supplies without shear stress on the cells with gradient device reaching space steps on the order of 100-300 mm. These are workable limits of normal microscopy and viability >90 percent when running on multiple days.
Is it possible to have PillarCell in co-culture with an organoid or a 3D culture?
Yes, two routes are common. Fate can be pre-patterned on pillars, which can then be transferred to 3D matrices, or pillars can be used to anchor thin layers of hydrogel, both topographically and biochemically. In either case, perfusion maintains a check on oxygen and metabolites without a complete bioreactor.
What are the primary engineering guardrails?
Flow stable biocompatible coatings; antibubble geometry; low drift pressure/ flow regulation; geometry (profilometry / SEM ) and flow (inline sensors) metrology. Catch the drift at an early stage by adding a simple verification panel – adhesion proteins, cytoskeletal reporters, and two members of the fate markers of each lineage.
So what is the place of the Microfluidics Innovation Center (MIC)?
On the spot, in the transfer of the idea to the tool. MIC not only designs the chip (pillar arrays + channels), but also incorporates closed-loop flow control and constructs verification benches to allow the reproduction of protocols outside the laboratory where they were originally developed. With this type of SME participation in EU consortia, and with our proposal-writing services (in addition), the success rates would approximate twice those of similar calls at official baselines.
What would it mean to be successful at the conclusion of the project?
An appropriate microscope-ready cartridge containing two or three proven pillar geometries, a plug-and-play perfusion controller, and a collection of SOPs providing the same differentiation shift in at least two laboratories. Such a combination of a lightweight data package (images, flow logs, and quantifying markers) would provide a portable means of reviewing the process that reviewers and partners can rely on.
I am developing a Horizon Europe consortium- where should MIC be placed outside this project?
MIC is a microfluidics SME specializing in regularly participating in EU consortia to provide hardware, automation, and measurement components for complex bioassays. We prepare offers together, model work packages based on prototype deliverables, and risk-proof manufacturable designs. Consortia incorporating MIC prototype-first model usually claim success rates that are twice the official baseline at similar calls- a trend we put down to obvious technical way, believable milestones, and early demonstrators.