A platform to engineer and optimize the behavior of biohybrid: Active Matter
Author
Audrey Nsamela, PhD
Publication Date
October 14, 2019
Status
Keywords
Micro- and Nano-Swimmers
microswimmer chemotaxis
biohybrid systems
self-propelled particles
catalytic degradation
catalytic propulsion
droplets
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Active matter is a new field of interest at the boundary between material science, chemistry, and cell biology.
A microfluidic platform will help study and characterize biohybrid active matter.
A platform to engineer and optimize behavior of biohybrid active matter: introduction
Active matter designates particles that show life-like features. They can be synthetic, biological, or a mix of both components.
These so-called micro- and nano-swimmers can convert the energy from their surroundings to propel themselves and react to external stimuli to reorient or create complex self-organized structures.
Studying active matter could lead to a better understanding of far-from-equilibrium physics and the dynamics at the origin of living organisms.
Many different propulsion mechanisms exist and are studied to reproduce the behavior of active matter.
Most of the time, it involves the catalytic degradation of a fuel (i.e., hydrogen peroxide) that creates a local electrolyte gradient that drives the particles.
In other systems, the propulsion is initiated by living cells, such as bacteria or sperm cells, that can be conjugated with inorganic particles.
In any case, many challenges remain before including these systems in real applications. For example, avoid the use of toxic fuels and increase the biocompatibility.
What is also lacking in most experiments is an external flow and boundaries around the particles that can affect the behavior of the active matter.
A platform to engineer and optimize behavior of biohybrid active matter: project description
Microfluidic systems are potent tools for performing flow and chemistry experiments. With its precise pressure controllers and flow sensors, we have the perfect setup to develop a microfluidic platform to study and characterize biohybrid active matter.
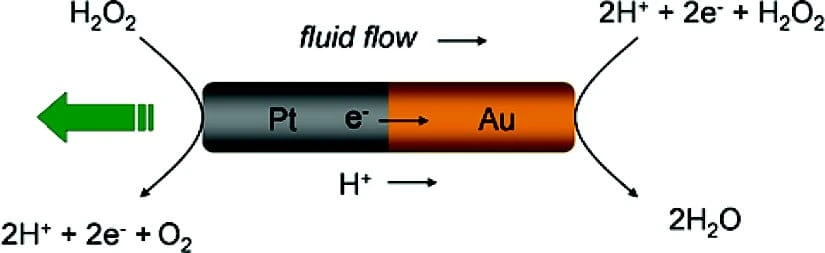
The microfluidic device will allow us to investigate the behavior of different types of biological microswimmers, i.e., various species of sperm cells, under flow conditions. Sperm cells’ motion is influenced by the topology of their surroundings and chemical, thermal, or viscosity gradients.
This project aims to take a step closer to real-life settings using active matter such as drug delivery, assisted reproduction techniques, sensors, etc.
Related content & results from this project
In the light of the Active Matter project, we developed a microbiology incubator.
We also published a review about soil-on-chip devices, and another one about microswimmers chemotaxis behavior.
Funding
This project has received funding from the European Union’s Horizon research and innovation program under the Marie Sklodowska-Curie grant agreement No 812780 (ActiveMatter project).
Researcher

Audrey Nsamela
PhD candidate Elvesys/Dresden University of Technology
- Master of Applied Science in Biomedical Engineering (Ecole Polytechnique Montreal, Canada)
- Master in Physics Engineering, oriented fundamental physics (Université Catholique de Louvain, Belgium)
- Bachelor in Engineering science, oriented applied physical chemistry and biomedical engineering (Université Catholique de Louvain, Belgium)
Areas of expertise:
Physics engineering, biomedical engineering, nanotechnology, surface functionalization, cell biology, immunoassay, in-vitro diagnostics, microfluidics, polymers.



Check our Projects
FAQ – A platform for the behavior of biohybrid: Active Matter
What do you mean by "droplet-encapsulated active matter"?
It is about self-propelled microscopic systems comprising bacteria, algae, and synthetic Janus particles that continuously feed themselves by dissipating energy to move or apply forces. We trap these agents in picoliter nanoliter drops of aqueous in oil droplets. The transport, signaling, and collective behaviors of a population consist mostly of isolated physiological bioreceptors with well-isolated droplets.
So what is the rationale for using droplets rather than conventional microchannels or bulk flasks?
Three reasons stand out. On the one hand, isolation: each droplet maintains a fixed composition, and at the same time, a fixed number (cells) of cells that addresses the common issue of population drift. Second, throughput: flow-focusing droplet generators regularly make 10³-10⁴ monodisperse droplets/second with a coefficient of variation of size that is usually less than 5, and are statistically robust to screens on a single run. Third, control: Surfactants, oil selection, and droplet geometry can be tuned to achieve motility, oxygenation, and mechanical constraints to a myriad compared to the bulk.
Which behaviours at all can be engineered or optimized within droplets?
Steering is possible to maintain single-cell behaviour (speed distributions, run-tumble bias) at low Reynolds number (Re ≪ 1), collective behaviour down to the vortices, and chemotaxis / phototaxis by ionizing a gradient field over the droplet. Using appropriate chemistries, we can also control quorum sensing, biofilm formation, and metabolic output (e.g., reporter fluorescence in response to pathway activity). Practically, optimization implies the translation of these readouts towards a quantitative goal, e.g., increased persistence length, reduced cost per unit of energy spent on displacement, reduced response time, or decreased variance between droplets.
How do droplets come into being and how are they maintained over a long period of time?
The majority of designs employ the flow-focusing / T-shaped geometry of PDMS or glass microchips. The stability must rely on: (a) interfacial tension and surfactant head-group, (b) oil phase (e.g., fluorinated oils where gas permeability is needed), and (c) Laplace pressure, inversely proportional to droplet radius. In case of overnight assays, we usually use a biocompatible fluorosurfactant (to prevent coalescence) and incubation chambers designed to reduce wetting defects and Ostwald ripening.
What are the ways to impose and determine gradients within a closed droplet?
Two routes are common. One applies embedded source/sinks, e.g., caged compounds dropped onto one side of the droplet and photo-released. The other is based on phase-transfer by the oil with the help of permeable species (oxygen, small hydrophobes) in such a way that a steady-state gradient develops across the diameter. Gradients can be measured using ratiometric fluorescence or biased trajectories; Péclet numbers are a simplified method of comparing designs with regard to advection vs diffusion.
So what is the constraint on the behavior of people in confinement?
Boundaries matter. Curved interfaces provide hydrodynamic trapping and reorientation as well as provide a natural length scale which competes with run length and interaction radius. Until larger droplets are operated in the presence of gas-permeable oils, nutrient and oxygen supply may be a rate-limiting factor. Lastly, motility is occasionally suppressed by surfactant toxicity, and returning to standard swimming speed with surfactant concentration and rinse regimens usually recovers baseline swimming speed over ±10%.
Is it possible to reuse droplets, which could be combined, split, and reprogrammed during an experiment?
Yes. Electrocoalescence of droplet pairs is performed at a specific address within a droplet to initiate treatment or provide predetermined conditions; dielectrophoretic sorting is used to generate daughter droplets with defined payloads in deterministic splitting, in applications such as biosensing and physical-biological control. It is through this that we execute closed-loop optimization: on a behavioral metric measure, update inputs (nutrients, chemoeffectors, light) and write the new condition back to droplets selected in the same device.
What is the way of measuring behaviors at large throughput without being overwhelmed by video data?
We combine grey- or fluorescence-based statistics with automated object tracking and detection to extract data on velocities, angular diffusion, vortex order parameters, and the spatial correlation length. Compressed acquisition with on-chip barcoding (size, dye ratio) allows labeling and analysis to remain manageable for screens with rates of droplets above 1 kHz. The result is normally a table of the description per droplet with confidence intervals calculated on thousands of replicates.
In which applications are the best applications to optimize the behavior of swimmers in droplets?
Three concrete ones:
• Strain engineering: choose according to motility gain or chemotactic gain which affects colonization or bioprocess yield.
• Synthetic active materials: mix, pump, or stir at the microscale without external actuators.
• Diagnostics and sensing: perfuse behaviors as a rapid detection of intoxicating levels, pathogen susceptibility to antibiotics, or metabolite concentrations, with decisions often possible in under 30 minutes.
In what ways does MIC usually work together in Horizon Europe-type consortia in this respect?
Our platform includes the microfluidic architecture (chip design, materials, bonding), high-throughput prototyping, and an automation layer, including droplet generation/sorting and imaging with a computational pipeline. Since we are an SME with a strong R&D track record, the involvement is likely to enhance proposal success rates: among multiple partner submissions in the recent past, consortia with MIC have been successful at roughly twice the official average. We also favor exploitation planning and early demonstrators, which are consistently rewarded by evaluators.