What is the HORIZON-HLTH-2026-01-DISEASE-04 Horizon Europe call?
Novel vaccines for viral pathogens with epidemic potential
Opening
10 February 2026
Deadline
Keywords
Cluster health
viral pathogens
epidemic potential
novel vaccine
clinical safety studies
health security
clinical translation
GMP readiness
immune profiling
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
HORIZON-HLTH-2026-01-DISEASE-04
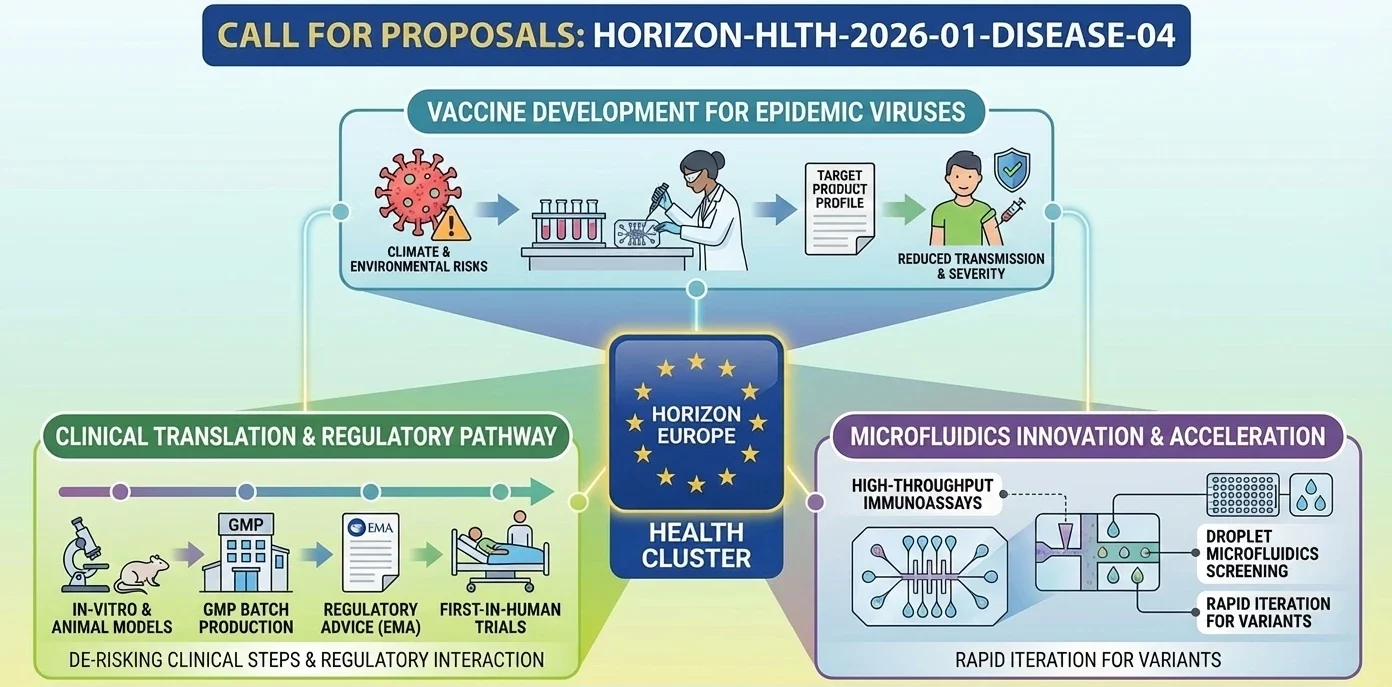
This topic is clearly identified in the Horizon Europe Work Programme 2026-2027 (Health, Part 4) as: “Development of novel vaccines for viral pathogens with epidemic potential”.
Discover more!
Administrative facts: what do we know about the HORIZON-HLTH-2026-01-DISEASE-04 call?
Which call is it under?
- Call name: Call – Cluster 1 – Health (Single stage – 2026)
- Call ID: HORIZON-HLTH-2026-01
What is the opening date and the deadline?
- Opening date: 10 Feb 2026
- Deadline: 16 Apr 2026
- All deadlines are at 17:00 Brussels local time (and dates may shift within the limits stated by the Commission services).
What about the budget and estimated size of the project?
- Type of Action: RIA (Research and Innovation Action)
- Overall topic budget: EUR 44.20 million
- Nominal number of funded projects: 5
- Budget/project: EUR 9-11 million
What are the evaluation thresholds you must hit?
- Thresholds: 4 for each criterion (Excellence / Impact / Implementation) and 12 cumulative.
Is there any special “portfolio” rule that changes the funding strategy?
- To ensure a balanced portfolio across the targeted viruses, funding is not strictly “ranking-only”: grants may also go to the highest-ranked proposals within each virus target, as long as they meet thresholds.
Deadlines of European Programmes
Download the MIC Horizon Europe 2026/2027 Calls Calendar:
-All Horizon Europe deadlines (by cluster and call).

Scientific range: what the Commission expects from the HORIZON-HLTH-2026-01-DISEASE-04 grant
What problem is the EU trying to solve here?
- The EU wants vaccine R&I that reduces preparedness gaps for viral pathogens with epidemic potential, in a context where emergence and spread risks are amplified (including by climate and environmental drivers).
- The expected endpoint is not a “paper-ready concept”: the topic is shaped to push candidates far enough to be credible for the next clinical steps.
What outcomes are expected?
- Safe and effective vaccine candidates should be developed for the targeted pathogens, advanced enough to proceed to larger clinical trials.
- Overall goal: accelerate vaccine development and increase preparedness, improving the ability to reduce transmission and disease severity during outbreaks.
How does the EU intend to structure the portfolio scientifically?
- Proposals must explicitly state which virus is targeted, and funding can be used to maintain coverage across different viruses (portfolio logic).
(Note: the Work Programme text also specifies a defined list of viruses and detailed R&D steps to cover, including in-vitro characterisation, relevant animal models, GMP batch production/scale-up, and first-in-human clinical safety studies.)
Scientific strategy: How can you enhance your chances of being funded through HORIZON-HLTH-2026-01-DISEASE-04?
How do you “look fundable” under DISEASE-04 (not just “scientifically interesting”)?
- Build your whole project around a credible vaccine development chain, not a fragmented set of experiments:
- Clear Target Product Profile logic (what “success” means for outbreak use).
- A development plan that reads like it can survive regulatory scrutiny and clinical translation.
How do you de-risk first-in-human and early clinical steps?
- Show you understand EU expectations for risk mitigation in first-in-human / early trials (dose rationale, stopping rules, integrated safety monitoring), and that your non-clinical package is designed to support that.
- Explicitly plan early scientific advice interactions (EMA or national competent authorities), and explain what you’ll ask them (endpoints, population, immunobridging strategy, comparators, etc.).
How do you turn the “portfolio rule” into an advantage?
- Because the EC can fund proposals to ensure virus coverage, you want reviewers to feel your project is:
- One of the best for a specific target virus, and
- A low-risk bet for portfolio balance (clear milestones, credible go/no-go, strong translation pathway).
Consortium & proposal-writing plan: what works best with this type of Health RIA?
Who should be “around the table” so reviewers trust the full pathway?
- Vaccine R&D excellence: antigen design, immunology, assay development, correlates-of-protection thinking.
- Preclinical capability: access to appropriate animal models, challenge expertise where relevant, and biostatistics.
- Translational + clinical: a team that can truly run first-in-human safety studies (clinical ops, pharmacovigilance, ethics, trial design).
- Manufacturing realism: GMP readiness and scale-up logic (even if via subcontracting/partners).
- Regulatory competence: someone who has “done this before” and can write a tight plan for interactions with regulators.
- Discreet but highly recommended: include an innovative SME (speed, execution, product mindset, and credibility on exploitation).
How should you write so evaluators can score you quickly?
- Mirror the Excellence / Impact / Implementation structure and make every subsection scorable.
- Use “reviewer-friendly mechanics”:
- One-page visual summary of the development pathway (inputs → work packages → decision gates → candidate readiness).
- Explicit go/no-go criteria and contingency plans (what happens if immunogenicity is insufficient?).
- A tight “Impact” narrative written for health security decision-makers, not only scientists.
How would microfluidics contribute to this topic?
- High-throughput immunoassays on-chip to accelerate neutralisation testing and immune profiling with lower sample volumes.
- Droplet microfluidics to accelerate antigen variant, adjuvant formulation, and antibody response screening.
- Quick, standardisation-capable analytical processes which enhance parity with partners (aids both “Excellence” and “Implementation”).
- Possible “EU preparedness” axis: microfluidic platforms can facilitate faster iteration if variants are identified, which aligns with countermeasures’ responsiveness in the medical setting.
The MIC already brings its expertise in microfluidics to Horizon Europe:
H2020-NMBP-TR-IND-2020

Microfluidic platform to study the interaction of cancer cells with lymphatic tissue
H2020-LC-GD-2020-3

Toxicology assessment of pharmaceutical products on a placenta-on-chip model
FAQ - HORIZON-HLTH-2026-01-DISEASE-04
What are the appraisal levels, and how does it work in real life?
Each of the criteria (Excellence, Impact, Implementation) must be scored at least 4/5, and the overall score must be 12/15. In practice, a single poor evaluation requirement can doom a good proposal, so the scientist (Excellence), the health-security policymaker/market shaper (Impact), and the project manager/regulator (Implementation) must all be represented well.
Does it fund based on rank or portfolio rule?
Explicit portfolio logic: to cover target viruses, the Commission can fund the best proposals in each area of selection, as long as there is a threshold. Translation: it is as important to be best-in-class for your preferred virus as it is to be ranked globally. Be clear about your decision and explanation of the virus.
What will be the scientific deliverables at the conclusion of the grant?
Protective and safe vaccine candidates that are developed to a degree where bigger clinical trials can be used. The work should include non-clinical packages of work with a coherent focus (e.g., in vitro characterization and pertinent animal models), GMP-scale-up production/batching of clinical material, where necessary, and first-in-human safety studies. The final product must be presentable as a regulator-ready product to the next step, rather than a prototype seeking a plan.
What should we do with our R&D plan to make it look fundable and interesting?
Ground the proposal on an evidence-based clinical translation path and an obvious Target Product Profile. Present dose rationale, stopping criteria, safety surveillance; match non-clinical trials to the target clinical population and clinical endpoint; present target decision-gate criteria that were measurable go/no-go. A pipeline diagram (1 page) that includes inputs, WPs, milestones, and readiness levels gives evaluators, at a glance, a sense of coherence.
In what ways can microfluidics be used to enhance a DISEASE-04 proposal?
Microfluidic systems are capable of condensing timeframes and minimizing sample sizes, such as in times of epidemics:
-On-chip neutralization and immune-profiling ranking of antigen/adjuvant options at higher throughput rates.
-Droplet microfluidics for rapid formulation screening and antibody response mapping.
-Automation-friendly, standardized, and reproducible workflows of analysis that enhance reproducibility across locations, as well as rapid data lock.
To put it into perspective, our team at the Microfluidics Innovation Center (MIC) designs end-to-end microfluidic systems, produces custom chips, and constructs prototype instruments on demand; these resources typically enhance the value of Excellence (vigorous science) and Implementation (rapidity of execution). Multi-partner consortia usually serve as a broker between immunology laboratories, GMP manufacturers, and clinical communities.
What consortium structure will score this RIA highly?
-Plausible chain of command between the bench and the bedside:
-Antigen design, immunology, assay development, and thinking about correlates of protection: vaccine R&D.
-Preclinical: access to proper animal models and challenge facilities; sound biostatistics.
-Manufacturing: collaborates with GMP preparation, process development, and scale-up reasoning (direct or subcontracted).
-Clinical: a group that is capable of conducting real first-in-human safety trials, taking into account such aspects as ethics, pharmacovigilance, and trial operations.
-Regulatory: A person who has already passed through EMA/national advice and initial trials.
An innovative SME: introduces speed, product attitude, and specificity of exploitation. Intuition suggests that introducing a specialized SME, such as MIC, into a Horizon Europe health consortium is much more efficient and, according to our internal monitoring, the odds of success are about twice as high as for comparable calls, as described in the official baseline reports.
Any writing strategies that can prompt evaluators to score quickly and generously?
Yes, reflect the Excellence/Impact/Implementation structure and create all subsections that can be scored. -Introduce reader-friendly mechanisms: a one-page graphical representation of the development pathway; clear and numeric go/no-go criteria (e.g., neutralization titer fold-rise laws, manufacturing productive capacity in each batch, equitable access); and an Impact section aimed at outbreak decision-makers (deployment turnaround, manufacturing responsiveness, fair access), rather than just academic colleagues.
What of TRLs, IP, data, and ethics is enough?
-Define your initial TRL, your desired final project TRL, and the change in the needle by each of your work packages.
-IP foreground/background and access rights map early to eliminate ambiguities during exploitation.
-Pledge to FAIR principles and safe management of clinical and genomic data, and a realistic data-management plan for data. The ethics areas are expected to address first-in-human safeguards, the inclusion of underserved populations for reasonable reasons, and dual-use/biosecurity.
What is the way to capitalize on the portfolio rule instead of being afraid of it?
Select an objective virus where your team is arguably best. Then, facilitate easy selection of the Commission to ensure equal distribution: demonstrate spotless milestones, schedule actual GMP and first-in-human studies, and outline a backup plan if an early candidate does not perform. And you would like people reviewing to think: “This is the surest bet in this area of viruses; they will come.”
What is the role of MIC with a DISEASE-04 project?
MIC excels in microfluidic and automation, providing design and manufacturing of custom chips, high-throughput immunoassays, droplet screening systems, instrument prototypes, and powerful analytical SOPs for partners. MIC entry success in recent consortia has nearly doubled the odds of success, despite the comparison of baseline acceptance rate and simple manufacturability and end-user validation rates, as these are accommodated during the very first month of consortium initiation and never tacked on in month 24.
Last-minute checklist- what should not be left out?
-The selection of the virus and rationale is evidently linked to EU preparedness.
-TPP has quantitative endpoints (immunogenicity, dose range, concentration range, manufacturing yield).
-GMP plan/ batch release testing strategy.
-First-in-human design synopsis, risk mitigation, and DSMB plan.
-Pre-regulatory-advice schedule and questionnaire.
-Go/no-go gates are numbered (Milestones).
-Exploitation route: scale-up, manufacturing partners, and route to pivotal trials.
-A one-page pipeline chart that any reviewer can read within 30 seconds.
