Tips & Tricks for a successful HORIZON-HLTH-2026-01-TOOL-03 proposal
New Approach Methodologies (NAMs) for biomedical research and regulatory testing
Opening
10 February 2026
Deadline
Keywords
Cluster health
New Approach Methodologies
RIA
regulatory testing
iPSC models
biomedical research
population variability
AI predictive models
NAMs
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
HORIZON-HLTH-2026-01-TOOL-03
This topic aims to speed up the shift toward human-relevant “New Approach Methodologies (NAMs)” by fully integrating them into both biomedical research and regulatory testing, from early discovery through clinical application and the regulatory assessment of medicines, medical devices, and also industrial/environmental chemicals, when relevant. It explicitly targets validated NAM solutions that can be adopted by industry and accepted by regulators, helping improve predictivity, increase trust, and ultimately reduce the use of live animals in research and testing.
Discover more!
Administrative facts: what do we know about the HORIZON-HLTH-2026-01-TOOL-03 call?
Which call is it, and when are the opening and the deadline?
- Call name: Cluster 1 – Health (Single stage – 2026)
- Call ID: HORIZON-HLTH-2026-01
- Topic: HORIZON-HLTH-2026-01-TOOL-03 Integrating New Approach Methodologies (NAMs) to advance biomedical research and regulatory testing
- Type of action: RIA (Research and Innovation Action)
- Opening date: 10 February 2026
- Deadline: 16 April 2026 (17:00 Brussels local time)
- Flexibility note (important in planning): the opening may shift by ±1 month and the deadline may be delayed by up to 2 months
What about the budget and estimated size of the project?
- Overall topic budget: EUR 49.00 million
- Indicative number of funded projects: 7
- Budget/project: EUR 5.00-8.00 million
Eligibility reminder specific to this destination: entities established in China are not eligible to participate as beneficiaries in RIAs and IAs under this destination
JRC involvement: the topic text explicitly encourages considering involvement of the European Commission Joint Research Centre (JRC), including EURL ECVAM for alternatives to animal testing
Scientific range: what the Commission expects from the HORIZON-HLTH-2026-01-TOOL-03 grant?
Core scientific intent
This topic is about making NAMs “real” for both science and regulation: not only developing impressive human-relevant platforms, but pushing them far enough in robustness, reproducibility, usability, and regulatory credibility that they can be adopted across biomedical research and accepted for regulatory testing.
What kinds of NAMs are explicitly in-scope?
The Work Programme frames NAMs as human-relevant approaches, such as:
- Advanced in vitro and ex vivo assays
- iPSC-based models, organoids, complex organ-on-chip (OoC) systems
- Human tissue models that better replicate physiology/pathology
- Complementary computational approaches like AI-driven predictive modelling and virtual twin technology (as part of increasing predictive power and clinical relevance)
- Embedded sensing for real-time monitoring (explicitly suggested)
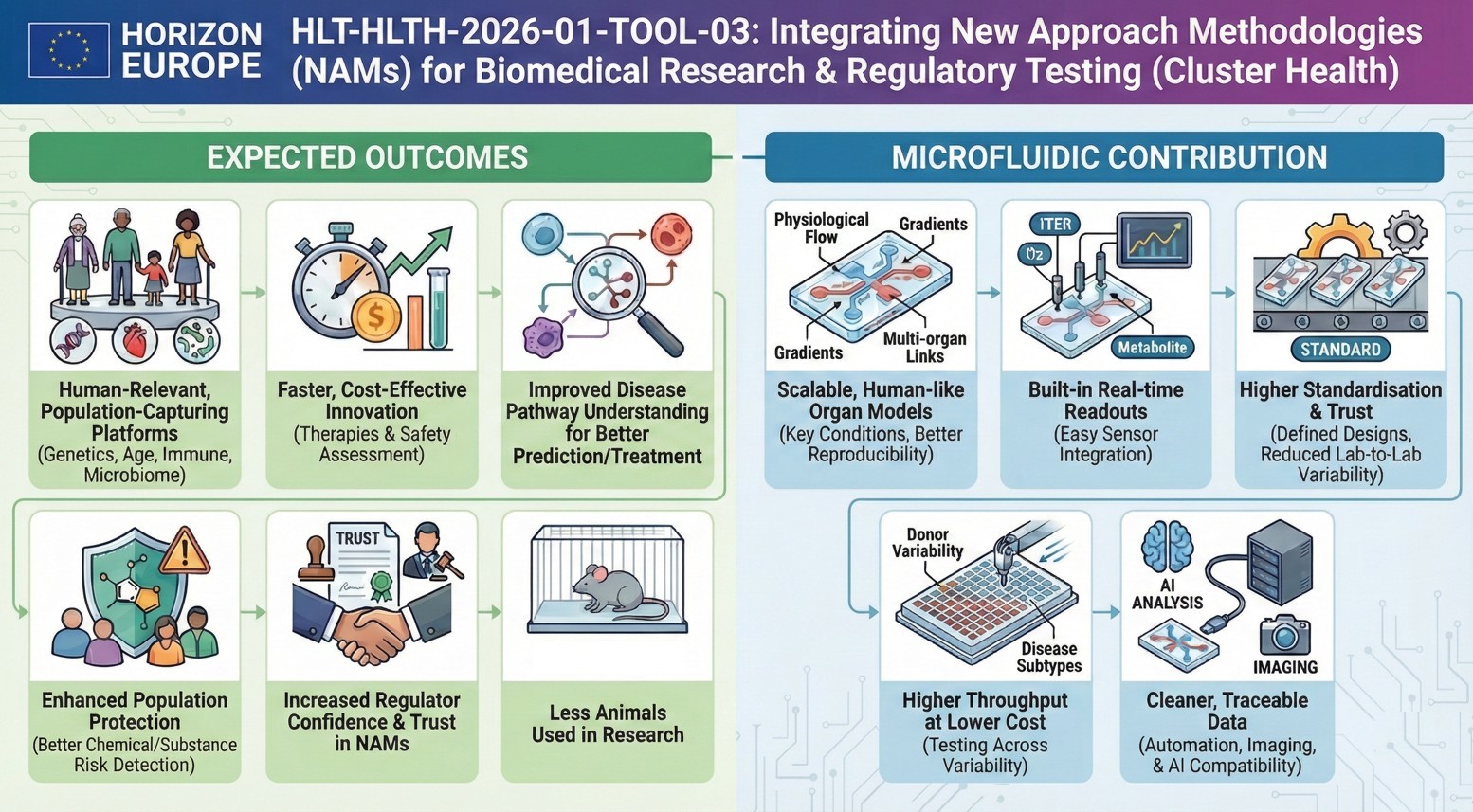
What outcomes does the Commission want to see?
The expected outcomes emphasise:
- Stronger, human-relevant platforms that capture population variability (genetics, phenotype, age, immune, microbiome, exposure)
- Faster, more cost-effective innovation for therapies and for safety assessment of chemicals/medical products
- Better prediction/prevention/treatment through improved disease pathway understanding
- Improved protection of the population via better detection/mitigation of chemical and substance risks
- Increased regulator confidence and trust in NAMs
- Fewer live animals used in research and regulatory testing
Cross-cutting requirements you must treat as non-negotiable
- FAIR data principles, data quality standards, interoperability, and GDPR-compliant sharing/access good practices
- Practical alignment with EU health data approaches (EHDS is referenced in the topic text) and relevant ESFRI infrastructures
- A cluster approach: all funded projects form a cluster and must participate in joint activities
Scientific strategy: How can you enhance your chances of being funded through HORIZON-HLTH-2026-01-TOOL-03?
What will evaluators silently look for in this specific NAMs topic?
- A credible pathway from “cool platform” to “accepted method”
- Define a context of use early (what decision will the NAM support, for whom, under what conditions?).
- Show how you will generate the evidence regulators/assessors need (repeatability, reproducibility, transferability, performance metrics, uncertainty).
- Scalability + reproducibility as first-class science
- If you propose OoC/organoid/iPSC systems: show how you will standardise protocols, materials, QC, and reduce lab-to-lab variability.
- Include an explicit plan for multi-site replication (not just “we will validate”).
- Population variability is not optional
- Build variability in by design (donor diversity, age/sex considerations, immune/microbiome/exposure factors) rather than as an afterthought.
- Real-time measurement is an advantage
- If you can integrate embedded sensing (physiological readouts, barriers, electrophysiology, metabolism), it directly matches what the topic text encourages.
- Use AI/virtual twins only where they are defensible
- If you include AI predictive models: emphasise curated datasets, bias minimisation, traceability, and interpretability.
- If you include virtual twins: make them operational (which endpoints, which clinical/research decisions, what validation strategy).
- Plan your regulatory interface like a work package
- Don’t hide it inside “dissemination”.
- Include structured interaction with regulators and reference bodies (and show how feedback changes your methods and evidence package).
- Data strategy that feels “EU-native”
- Explicitly describe how you will curate, standardise, and share data under FAIR, with governance and GDPR compliance
- Don’t just say “we will follow FAIR”: show concrete standards, metadata, repositories, and access models.
A very practical “topic-fit” checklist
- Does the proposal clearly cover both:
- biomedical research usefulness and/or regulatory testing relevance, and
- a realistic maturity trajectory (from TRL-like prototype → robust, replicable platform → accepted use)?
- Do you show why current animal models are insufficient for your exact use case, and how your NAMs improve human relevance?
- Have you budgeted and planned the cluster participation activities (joint meetings, shared dissemination, joint reporting), since clustering is required?
Consortium & proposal-writing plan: what works best with this type of Health RIA?
What consortium shape tends to “fit” this kind of EU NAMs RIA?
- Academia + applied R&D + infrastructures + regulators at the same table
- Academia: mechanistic depth + disease biology
- Tech developers: platforms, sensors, data pipelines
- Research infrastructures: harmonisation, data standards, access, interoperability
- Regulators/reference labs: acceptance pathway, evidence expectations, context-of-use framing
- Include industry, but not just as a “user”
- Industry should co-define performance requirements, scalability constraints, and adoption barriers.
- Discreet but strong recommendation: include at least one innovative SME that actually owns/advances a NAM enabling technology (chips, sensors, automation, computational models). It helps credibility on exploitation and speed-to-adoption.
- Consider engaging the JRC / EURL ECVAM angle where relevant, since the Work Programme explicitly points to it.
Writing tactics that score under Excellence / Impact / Implementation
- Excellence
- Write your “scientific bet” in one sentence: what exactly becomes possible that is not possible today?
- Define measurable performance endpoints (precision, sensitivity, reproducibility, throughput, cost/time savings).
- Impact
- Name the adoption pathway: who will adopt, when, under what constraints, with what evidence package.
- Show that your outputs can be reused beyond the consortium (standards, SOPs, reference datasets, open protocols where possible).
- Implementation
- Make validation and replication a visible backbone (WP structure + milestones + go/no-go criteria).
- Show cluster participation as a planned asset (shared dissemination, shared standards, avoiding duplication).
- Compliance hygiene
- Explicitly address FAIR + GDPR operationally.
- Remember eligibility constraints (e.g., China beneficiary restriction under this destination).
How would microfluidics contribute to this topic?
Microfluidics is almost “made for” this topic because it turns NAMs into controllable, repeatable, scalable systems:
- Organ-on-chip at scale
- Microfluidic chips enable stable perfusion, gradients, shear stress, multi-organ coupling, and long-term culture, key for human relevance and reproducibility.
- Embedded sensing, naturally
- Microfluidic platforms integrate sensors (TEER, oxygen, metabolites, electrophysiology), aligning with the topic’s push toward real-time monitoring.
- Standardisation potential
- Chips + defined flow + defined materials can reduce variability across sites, exactly what you need for inter-lab replication and regulator confidence.
- High-throughput + lower cost per condition
- Parallelised microfluidic arrays help explore population variability (donor panels, disease subtypes, exposure scenarios) without exploding budgets.
- Cleaner data pipelines
- Microfluidics pairs well with automated imaging and AI-based analysis, improving traceability and dataset quality, useful when you claim bias-minimised, curated training data.
The MIC already brings its expertise in microfluidics to Horizon Europe:
H2020-NMBP-TR-IND-2020

Microfluidic platform to study the interaction of cancer cells with lymphatic tissue
H2020-LC-GD-2020-3

Toxicology assessment of pharmaceutical products on a placenta-on-chip model
FAQ - HORIZON-HLTH-2026-01-TOOL-03
What is HORIZON-HLTH-2026-01-TOOL-03?
It is an RIA call that focuses on the implementation of New Approach Methodologies (NAMs) in biomedical research and regulatory testing. The objective is to replace animal testing with human-relevant platforms throughout the entire innovation pipeline, including early discovery and regulatory evaluation of medicines, medical devices, and chemicals.
Deadline and budget of the call?
The call will be opened on 10 February 2026 and will have a deadline of 16 April 2026 17:00 Brussels time. The overall budget is EUR 49 million, which is likely to finance about 7 projects, each with a budget of EUR 5-8 million. Refer to the fact that the opening can be changed by +-1 month and the deadline can be extended by 2 months.
What are New Approach Methodologies (NAMs)?
The NAMs include human-relevant methods such as complex in vitro and ex vivo models, iPSC-based models, organoids, organ-on-chip models, human tissue models, AI predictive modeling, virtual twin technology, and embedded real-time monitoring and sensing. It is interested in methods that are more realistic and relevant to human physiology and pathology than traditional animal-based models.
What are the most important anticipated outcomes?
Projects are expected to provide human-relevant systems that include population variability, accelerated and cost-efficient innovation, enhanced disease pathway knowledge, improved chemical risk detection, enhanced regulatory confidence on NAMS, and improved animal use in research and test. More importantly, it must be shown that the results are not only scientifically excellent but also accepted by regulators.
What is the requirement of cluster approach?
Each and every funded project must be part of a compulsory cluster that involves joint activities, common dissemination, synchronized reporting, and collaborative standard-setting. The suggestion should also plan and budget for cluster participation, as this is not an optional feature.
Why is a consortium competitive in this topic?
Powerful consortia include academia (mechanistic depth), technology developers (platforms and sensors), research infrastructures (harmonization and standards), and regulators or reference laboratories (acceptance pathways). The involvement of industry is essential, but must extend beyond end-user involvement to define performance requirements and the impediments to adoption. The credibility of exploitation is enhanced by incorporating innovative SMEs with technologies that enable NAM.
What should be proposed in regard to regulatory acceptance?
Do not consider the regulation interface as a mere dissemination. Identify early context of use (what decision, to whom, under what conditions), organize interactions with regulators and reference bodies, and demonstrate how regulatory feedback will influence methods and evidence packages. Incorporate articulate multi-site replication plans that indicate repeatability, reproducibility, and transferability.
What are the essential data and compliance issues?
The proposals need to operationalize FAIR principles of data using tangible standards, metadata, repositories, and access models. demonstrate compliance with GDPR requirements, adherence to EU health data approaches (EHDS is mentioned), and compatibility with appropriate ESFRI infrastructures. Present data governance, which is perceived to be EU-native, and not generic compliance statements.
What is the role of population variability in proposals?
The variability of the population should not be an afterthought; it should be built in. The proposals must cover the diversity of donors, age and sex factors, immune systems, microbiome, and exposure conditions. Such variability can be investigated cost-effectively using high-throughput methods, such as parallelized microfluidic arrays.
What is the application of microfluidics in this topic?
Microfluidics enables controllably perfused, gradient-based, and multi-organ-coupled organ-on-chip systems to be more human-relevant. They inherently incorporate sensors to monitor in real time, minimize inter-labor differences through uniform protocols, support large-scale population investigations, and generate clean data for AI analysis. These features specifically address the issues of reproducibility, scalability, regulatory credibility, and real-time physiological measurement, as emphasized in the topic.
