Automated Perfusion System for Calcium Imaging
Control solution exchange in real-time calcium imaging experiments
Real-time monitoring
Track immediate cellular responses to calcium changes
Sequential compound delivery
Introduce reagents in a controlled sequence
Minimal sample disturbance
Maintain cell viability with gentle handling

Need a microfluidic SME partner for your Horizon Europe project?
How our solution enhances calcium imaging
In live-cell calcium imaging experiments, timing is crucial when introducing different solutions to observe intracellular calcium flux. Manual switching introduces inconsistencies that can distort results and compromise data accuracy [1].
Our microfluidic-based automated perfusion system eliminates this problem by delivering precisely controlled calcium influx and efflux cycles.
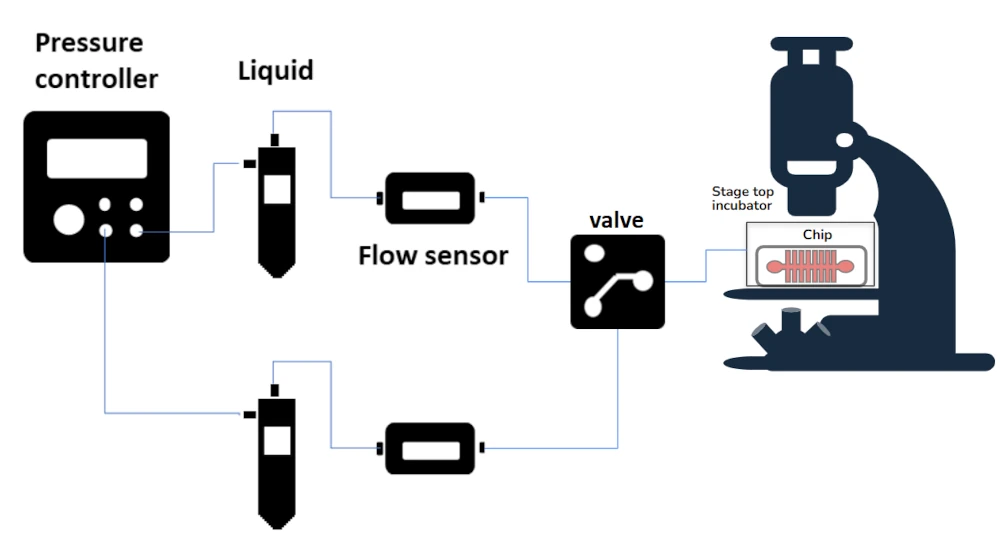
Researchers can study real-time calcium signaling in immune cells, neurons, and cardiac tissues with improved accuracy [2]. The system is also ideal for high-throughput screenings of calcium channel blockers and other drugs, facilitating drug discovery and pharmacological research [3].
In addition, it maintains controlled microenvironments for long-term imaging experiments, ensuring optimal cell viability. By eliminating timing discrepancies, this system enhances reproducibility across experiments, leading to more consistent and reliable data [4].
This system also prevents sample contamination and minimizes reagent waste, making it a cost-effective and high-precision solution for laboratories working on calcium imaging [5].

References
Zhang, Y., et al. (2023). A Programmable Microfluidic Platform to Monitor Calcium Dynamics in Microglia during Inflammation.
Taylor, A. M., et al. (2005). A microfluidic culture platform for CNS axonal injury, regeneration and transport.
Van der Linden, A., et al. (2006). High-throughput screening for N-type calcium channel blockers using a scintillation proximity assay.
Whitesides, G. M. (2006). The origins and the future of microfluidics.
Sigma-Aldrich. (n.d.). Live Cell Imaging Analysis.
Other applications
Our automated perfusion system is not limited to calcium imaging- it can also be used in:
- Electrophysiology studies for ion channel research
- Neuroscience experiments requiring controlled neurotransmitter applications
- Cardiovascular research for studying calcium-driven cardiac contractions
- T-cell activation and immune response studies in microfluidic environments
- And many more!
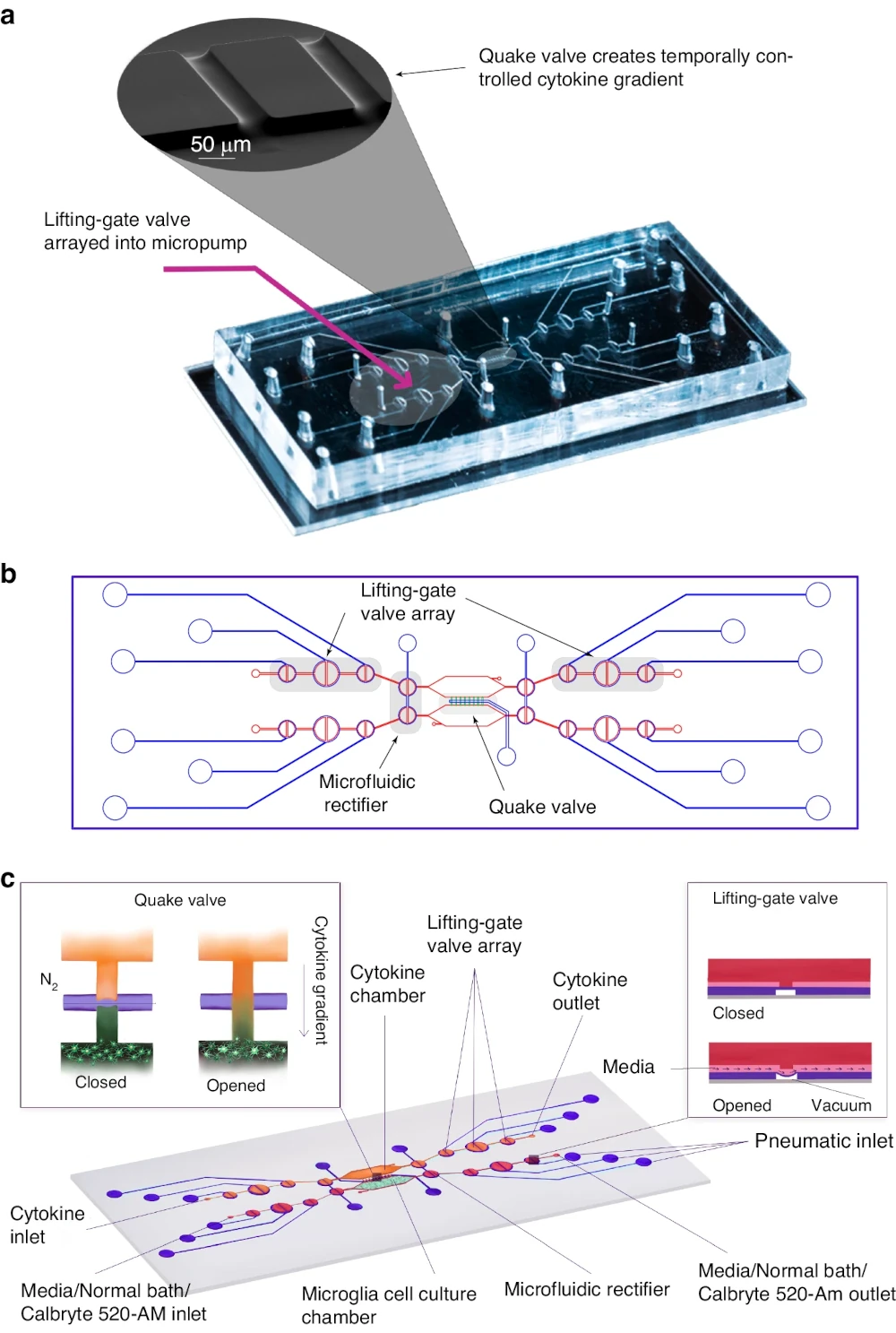
Calcium imaging in microfluidic systems: This figure illustrates a microfluidic platform designed for high-throughput calcium imaging of neuronal activity. Such systems enable precise environmental control and real-time observation of intracellular calcium dynamics, which are crucial for understanding neural responses. The integration of microfluidics with advanced imaging techniques exemplifies the growing potential of lab-on-a-chip platforms in neuroscience research.
References
Image :
Ma, Y., Li, J., Zhu, J. et al. A high-throughput microfluidic platform for calcium imaging of 3D neuronal cultures. Microsystems & Nanoengineering 10, 33 (2024).
Perfusion pump technical specifications for Calcium imaging
| Pressure control | |
|---|---|
| Pressure range | -400 to 600 mbar |
| Pressure stability | 0.2 mbar |
| Air flow rate | 0.1L/min at atmospheric pressure Possibility to work with higher air flow rates by reducing the pressure range |
| Flow control | |
| Microfluidic flow sensor | Monitoring and feedback loop flow control available |
| Flow rates | From 0.1 µL/min to 5 mL/min |
Stage top Incubator specifications for Calcium imaging
| Characteristics | Specifications |
|---|---|
| Dimensions (mm) | 30.5 x 130 x 168 (h x w x l) |
| Base K- Frame | 3.5 x 110 x 160 (h x w x l) |
| Dimensions of internal usable space | 25 x 89 x 130 (h x w x l) |
| Dimensions of the bottom glass (ITO glass) | 1) 72 x 110 with a thickness of 1.1 mm 2) 50 x 25 with a thickness of 0.6mm 3) 50 x 22 with a thickness of 0.12 mm |
| Temperature range | Room Temperature to 70 °C |
| Temperature accuracy | ± 0,5 °C |

Frequently asked questions
Can the solution exchange timing be adjusted?
Yes, the system allows full customization of perfusion timing to match your experimental protocol.
Is this system compatible with existing microscopes?
The stage top incubator fits a k-frame microscope.
Can it be used for drug testing?
Absolutely. The system is ideal for high-throughput screening of calcium-modulating compounds.
How can we help your experiment?
This pack is in beta testing phase. So, although the instruments are not fully industrialized, we can provide extensive support as part of our beta testing program. Get in touch to see if you are eligible.


Funding and Support
This project has received funding from the European Union under HORIZON-EIC-2022-PATHFINDEROPEN-01-01, grant agreement No. 101099719 (THOR).
Products & Associated Accessories
FAQ - Calcium imaging
Calcium Imaging Pack Contents.
Fundamentally, it is an automated perfusion system comprising of:
A pressure-driven control of perfusion, i.e. a pressure-driven control instead of gravity switching.
A microfluidic flow sensor (with monitoring and optional feedback loop).
A stage-top incubator that was to be placed on a microscope (K-frame format).
Is the flow and pressure control accurate (numbers not vibes)?
The following are the crucial figures to this setting:
Pressure range: -400 to +600 mbar
Pressure stability: 0.2 mbar
Air flow rate: 0.1 L/min at atmospheric pressure (greater rates can be used provided you decrease the range of pressure)
Liquid flow rate range (with sensing/control): 0.1 uL/min -5 mL/min.
These figures are significant in that calcium imaging tends to be very sensitive to minor inconsistencies- particularly when attempting to compare kinetics across days, users or in different conditions.
Is it possible to program complicated sequences of perfusion (not just A then B)?
Yes. The system features sequential compound delivery and is explicitly designed to be fully customised to your perfusion timing protocol. Practically, that allows scheduling pulses, wash steps, ramps (through timing/sequence design), and multi-reagent processes without the customary someone standing by the bench with tubing.
What is the best way to use this pack in experiments?
The headline is calcium imaging, which, however, has more uses whenever timed solution exchange is of concern:
Calcium signaling (fast activation, repeated stimulation) of immune cells.
Calcium physiology (calcium kinetics and calcium consistency) in the neurons and cardiac tissue.
Calcium-modulating compound (e.g., channel blocker) high-throughput screening.
Can it be used with my microscope and live-cell imaging workflow?
The incubator in this pack has stage-top design to fit K-frame microscope. In case your imaging platform is compatible with K-frame, then it is normally easy to integrate. When you have something that is non-standard (presumably, custom stages, bizarre goals, bizarre clearance constraints) it is normally an integration question but not the baseline of the K-frame, but the K-frame is the anchor point.
What are the stage top incubator specifications (size and temperature)?
Some of the important specifications of the incubator include:
Total size (h x w x l): 30.5 x 130 x 168mm.
Base K-frame (h x w x l): 3.5 x 110 x 160 mm
Internal usable space: 25 x 89 x 130 mm.
None, or as required: Bottom glass (ITO glass):
72 x 110 mm, thickness 1.1 mm
50 x 25 mm, thickness 0.6 mm
50 x 22 mm, thickness 0.12 mm
Temperature range: Room temperature up to 70 °C.
Temperature accuracy: +-0.5 °C
The final line (+-0.5 °C) may be more applicable than the highest temperature as drift may mimic biology when a long imaging session is conducted.
Is it useful in terms of reproducibility or is that marketing jargon?
Reproducibility is the area where automation is likely to be the most quietly useful. With a scripted exchange schedule, flow tracked (and optionally fed back) you minimize variation between run-to-run caused by human operator switching, inaccurate tube positioning, and small bubbles or backpressure events. On a dataset, that usually is manifested by tighter response distributions and less of the so-called mystery outliers, particularly in kinetic analysis. Much of this reasoning can be generalized to more microfluidics reproducibility arguments: Whitesides, 2006).
Is the pack available to procure on a routine basis or is it experimental?
It is at a beta stage. Translation: the instruments are not yet exposed as being fully industrialized, but the trade-off is more than usually hands-on assistance in the beta program. In some cases, which are the advantages of this to labs: you get a say on features, and quicker troubleshooting; in other cases it can be nothing more than a governance choice.
My Horizon Europe proposal is being constructed- why not use microfluidics SME to do calcium imaging?
Because the reviewers do not simply score the science, they also assess the credibility of implementation, risk handling, and exploitation. A microfluidics SME partner can de-risk integration (chips + fluidics + imaging constraints), expedite prototyping, and help you make a case for reproducibility and scalability that you cannot hand-wave. MIC is an R&D partner: Microfluidic setup design for challenging research, microfabrication of microfluidic chips, automation of microfluidic flow control, and a prototype capable of withstanding real laboratory environments.
The particular calcium imaging project is also connected with EU funding under HORIZON-EIC-2022-PATHFINDEROPEN-01-01 grant agreement No. 101099719 (THOR) which may be of use as a context when composing impact and credibility sections.

