Organ-on-chip for personalised biomaterial risk assessment: PANBioRA
Author
Christa Ivanova, PhD
Publication Date
January 25, 2018
Status
Keywords
cytokine profiling
cytotoxicity testing
Organ-on-chip
predictive modeling
biomaterial risk assessment
biomaterials engineering
modular assay platform
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Biomaterial risk assessment: introduction
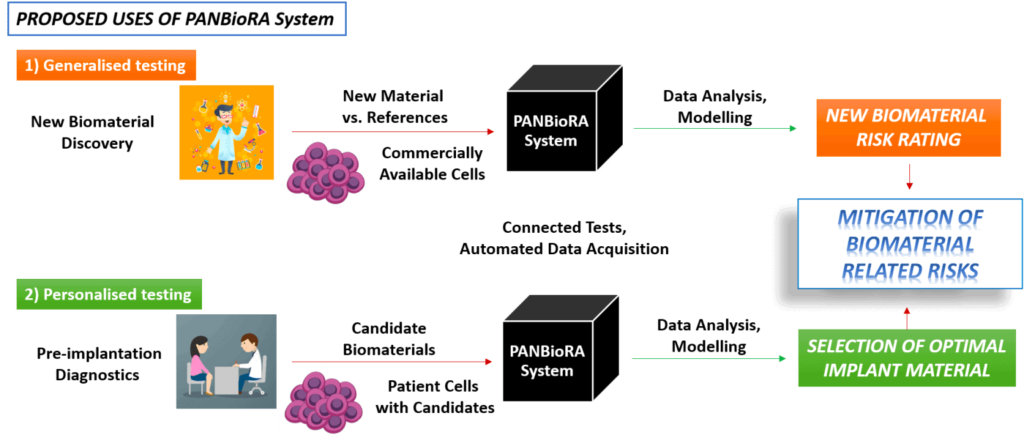
The goal of PANBioRA is to provide a set of tools to standardize the evaluation of new biomaterials.

Lots of medical devices, like implants, coronary stents, or fracture pins, are made of biomaterials.
The biological evaluation of new biomaterials is currently time and resource-consuming.
Moreover, once implanted, biomaterial devices often lead to complications such as inflammation or infections, showing the difficulty in assessing their innocuity and the importance of developing new tools for personalized pre-implantation diagnostics.
PANBioRA aims to provide a set of tools to standardize the evaluation of new biomaterials.
The same platform should also allow personal testing of different materials to assess the risks and choose the most appropriate for each patient.
We will share our expertise in microfluidics with this consortium of 17 partners spread across 11 countries to help develop this new set of tools.
Biomaterial risk assessment: our role
The biomaterials risk assessment will be based on standardized genotoxicity and cytotoxicity tests implemented at a microfluidic scale. The MIC is in charge of implementing a user-friendly, compact microfluidic flow control system that allows the controlled perfusion of i) organ-on-a-chip that mimics the critical barriers of the body and ii) standard on-chip cultures for cell-based cytotoxicity tests.
The microfluidic system will allow cell monitoring by conveying the supernatants from the devices to appropriate sensors to offer a read-out with quantifiable values of the toxicity of a biomaterial, the latter being developed by other partners.
Additional refined, miniaturized versions of existing genotoxicity methods are also controlled using Elveflow proprietary systems and in-house know-how.
For the PANBioRA project, we work with OEM custom fluidic system (Elveflow) to do the pressure management of different solutions in a reduced space and with LabViewTM libraries for higher compatibility and integration with the rest of the system.
Related content
In the light of this project, we have published a review on liver-on-chip technology.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 760921 (PANBIORA project).


Check our Projects
FAQ – Organ-on-chip for personalised biomaterial risk assessment: PANBioRA
What was PANBioRA in one line?
Creating an additive structure that will predict the response of a patient and individual organs to a particular biomaterial prior to implantation through integrating microfluidics, organ-on-chip, immunological, and computational risk analysis.
What is the purpose of having a new risk-assessment method for biomaterials?
Classical biocompatibility tests can be based on generic cell lines and static assays. That lacks significant variables, immune idiosyncrasies, organ-specific toxicity, microbiome interactions, and coupled tissue impacts. This was addressed by PANBioRA through swings in both directions, from antibody responses and cytotoxicity/genotoxicity in integrated tissues-on-a-chip to system-level modeling.
What are the modules of the PANBioRA platform?
Four pillars, which are intended to be interlocked:
Profiling of immune/antibody response to prevent sensitization and adverse reactions.
Cell-level cytotoxicity/genotoxicity using automated image analysis.
Organ-on-chip models that include local and systemic effects (the reference tissues used were respiratory epithelium, gut, and liver).
Physicochemical/biomechanical characterization and predictive modeling (e.g., risk radar and simulations of hard-to-test situations, including multifaceted interactions between biomaterials and microbiota).
What is the meaning of personalised in this context?
Two routes. To start with, the immune and cell-level assays can be fed with patient-specific cells or sera. Second, organ-on-chip tissues can be perturbed to simulate disease-relevant conditions (e.g., an inflamed gut or a steatotic liver). Combined, these provide a patient-conditioned readout rather than a one-size-fits-all distinction between biocompatible/not biocompatible.
What are the organ-on-chip components that increase the level of realism?
Microfluidic flow creates physiological levels of shear, gradients, and barrier function not achievable in static wells. The respiratory, gut, and liver chips allow you to see target-organ action and crosstalk; module connection allows you to record local reactions that become systemic.
What was MIC (Microfluidics Innovation Center) actually building?
MIC designed the pressure-driven microfluidic control layer, compact OEM microfluidics hardware, for constant, bubble-free flow; bubble-free plumbing in small spaces; and control software (LabVIEW-compatible libraries), so the organ-on-chip modules slide neatly into the rest of the test bench. In brief, we have ensured the right flows in the right order each time in biology.
What comparison does PANBioRA have with traditional ISO biocompatibility workflows?
It complements them. The regulatory basis is the ISO tests, supplemented by patient conditioning and organ specificity in PANBioRA. In practice, this would imply prior identification of issues (e.g., immune sensitization or liver-specific toxicity) and a more solid foundation on which to base material selection without lengthy in vivo cycles.
What type of outputs does the platform provide to the decision-makers?
Individual, module-level results (e.g., titers of antibodies, integrity of barriers, cytotoxicity score) and an overall risk radar of how likely/severe all the endpoints are. The point is to inform design decisions, alter a surface finish, replace a polymer, or stratify patients when time permits.
What is the rationale of having a dedicated SME such as MIC in a biomaterials or organ-on-chip proposal in Horizon Europe?
Since implementation transforms ideas into facts. MIC also integrates microfabrication, fluidic automation, and integration in a single roof-top, with a history in EU consortia. In our case, proposal chances are approximately twice as high when MIC is embedded as a microfluidics WP than when using official calls as a control group, and the time to get working prototypes to the validation stage is shortened.
What can MIC offer next in case a consortium intends to continue with PANBioRA?
An end-to-end flow-optics-sensing package: chip design and microfabrication; small-scale, multi-line pressure measurements; on-line sensing and quality control; computer software integration with existing imaging and analysis; and instrument-quality prototypes with documentation to scale-up and regulatory standards.