Artery-on-chip to target vascular diseases: CAR-OAC
Author
Oore-ofe Olumuyiwa Akeredolu, PhD
Publication Date
February 11, 2020
Status
Keywords
Atherosclerosis
Artery-on-chip
carotid artery disease
vascular diseases
thrombosis modelling
clot formation
drug testing
vascular pathology
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Strokes remain the second leading cause of mortality, the third for disability, and a top contributor to dementia and depression globally.
To date, existing tools are insufficient to target preceding vascular pathology, leading to its progression to a critical state, particularly carotid artery occlusion.
Carotid artery-on-chip to target vascular diseases: introduction
Subtle changes of gradual carotid occlusion put the entire cerebral vasculature at risk of an ischemic event.
Suboptimal monitoring leads to a late discovery of vascular atherothrombosis, usually only after an ischemic event.
Current research focuses on the effects of atherothrombosis on cerebral perfusion.
They study how plaques and emboli create temporary occlusion, leading to a transient ischemic attack or emboli dislodge from wider carotid arteries and relodge in narrow cerebral arteries, which causes an ischemic or hemorrhagic event. This could be better observed with the help of an artery-on-chip.
Current treatments mainly focus on individuals already affected by cardiovascular diseases caused by such changes.
To bridge this gap between the rapid deterioration of vascular pathology and the temporary or total occlusive events in the carotid artery, microfluidic technology can serve as a platform to model deteriorating vascular physiology with a carotid artery-on-chip.
This technology could provide a platform to investigate clinical interventions besides thrombectomies while providing a tool to develop prophylactics for a wide range of individuals who are anatomically at risk but may not yet show disease symptoms.
Microfluidic organ-on-chip engineering has enabled the optimization of microscale instruments that can mimic physical and biological changes to vascular cells that could contribute to occlusive pathology [1, 2], which makes the development of an artery-on-chip possible.
The influence of shear forces, rheological changes, and varied physiological conditions can be studied on cellular and molecular scales in an environment closer to nature than classical 2-D cell cultures.
Ultimately, this microfluidic device may detect occlusion and investigate the actions of drugs of interest on vascular atherothrombosis while helping to develop clinical interventions that help maintain an unrestricted blood flow in carotid vessels.
Carotid artery-on-chip to investigate thrombotic and atherothrombotic events: project description
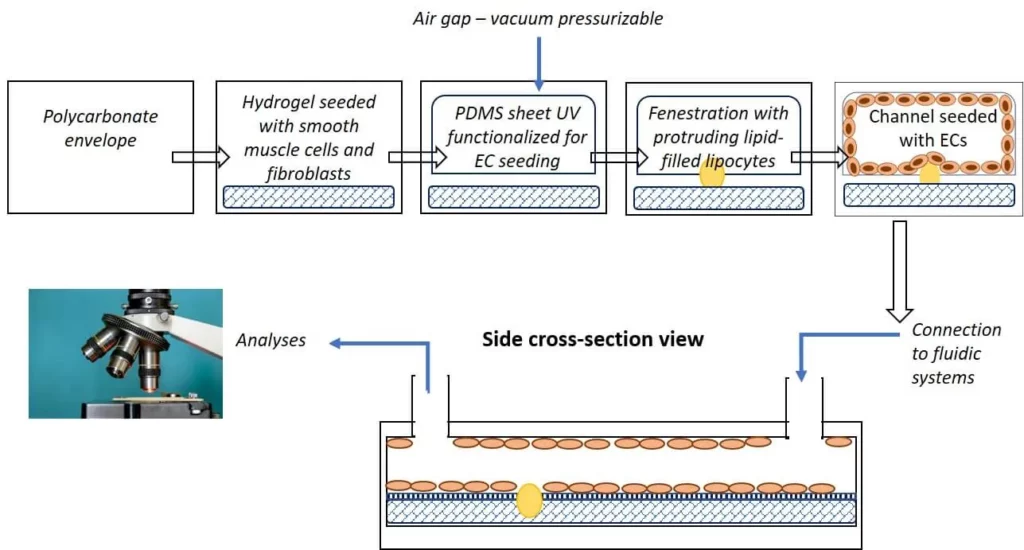
The goal of this artery-on-chip project is to develop a detailed and representative microfluidic organ-on-chip device that combines the primary biophysical, biological, and molecular cues that are typically found in carotids, which are starting to undergo occlusive atherothrombotic pathology.
Platelets, the leading cellular influencers of thrombotic events, can be introduced into the unit to examine their associations with the endothelial cells and fatty deposits.
Flow conroller and bubble traps with their respective components (Elveflow) will be integrated to optimize the lifespan of cells in the vascular atherothrombosis mimetic device, which will be the artery-on-chip. To see the results of this project, make sure to read this review written by Oore-Ofe.
References
- Greineder CF, Johnston I, Villa CH, Cines DB, Poncz M, Muzykantov VR. A Microfluidic Model of Microvascular Inflammation: Characterization and Testing of Endothelial-Targeted Therapeutics. Blood. 2015;126(23):3454-3454.
- Griffin MT, Kim D, Ku DN. Shear-induced platelet aggregation: 3D-grayscale microfluidics for repeatable and localized occlusive thrombosis. Biomicrofluidics. 2019;13(5):054106.
This project has received funding from the European Union’s Horizon research and innovation program under the Marie Sklodowska-Curie grant agreement No 843279 (CAR-OAC project).
Researcher

Oore-ofe Olumuyiwa Akeredolu
Post-doctoral Fellow
- Doctor of Philosophy in Physiology (University of Pretoria, South Africa)
- Master of Science in Biomedical Sciences (University of North Texas Health Science Center, Fort Worth, USA)
- Bachelor of Science in Biology with a minor in Chemistry (University of Houston, Houston, USA)
Areas of expertise:
Advanced cellular imaging (using light-, electron-, fluorescence-, and atomic force microscopy), platelet and red blood cell structural biology, disease-induced thrombosis.


Check our Projects
FAQ – Artery-on-chip to target vascular diseases: CAR-OAC
What is the clinical issue that CAR-OAC is attempting to address?
Gradual, progressive carotid artery constriction may remain silent until a transient ischemic accident happens or a complete ischemic stroke takes place. Strokes are the second and third major causes of death and disability, respectively, all over the world. CAR-OAC is a laboratory model of human-induced carotid segment deterioration: it is controllable and allows investigation of the mechanics of atherothrombosis before catastrophic events occur.
Why is the carotid artery a good example to model a carotid artery on a microfluidic chip as opposed to a regular cell culture?
Since the disease is determined by hemodynamics, platelet adhesion, plaque destabilization, and the formation of emboli depend on shear stress, pulsatility, and local geometry, which are difficult to replicate in flat, stationary cultures. A microfluidic artery-on-chip recreates these signals with physiological flow and materials that enable endothelial cells to function in vivo.
What is it in particular that the chip recreates?
Three cue layers are designed together (i) biophysical (managed shear and pressure gradients), (ii) biological (endothelial lining and fatty deposits interaction), and (iii) molecular (pro and anti-thrombotic signaling). Platelets may be perfused to observe the manner in which they aggregate during conditions of stenosis and the mechanism of micro-occlusions formation and dissolution.
What is done to ensure that flow quality is maintained when making experiments?
The platform operates on a precision pressure-driven controller that has inline bubble traps and related fittings. The actuation to steady flow and the control of bubbles to cell viability and spurious occlusions, required to observe platelet-endothelium dynamics on tens-of-minutes scales, are provided by pressure actuation and bubble management, respectively.
What are the default measurements that CAR-OAC can provide?
Common readouts include time-to-occlusion at a specified shear rate, platelet adhesion density, ramped-shear clot stability, and endothelial responses (e.g., barrier integrity markers). Since the flow can be programmed, pre-occlusive conditions, transient events during embolisms, and recovery can be tested during the same test run.
What is its position in the research arena?
Organ-on-chip models have advanced to incorporate microvascular inflammation and shear-induced platelet aggregation in a repeatable manner. Those concepts are generalized to a carotid-scale hemodynamic regime, and CAR-OAC generalizes thrombotic mechanics to an anatomically inspired geometry rather than a generic microchannel.
How is it to be used, i.e., discovery, screening, or preclinical validation?
All three. Discovery research is geographic discovery on the effect of stenosis severity on thrombus kinetics; screening studies on the comparative properties of anti-thrombotic candidates under different controlled shear conditions; and mechanism studies on how and why emboli form, dislodge, and re-lodgement downstream. The chip can similarly be used to develop a prophylaxis strategy in at-risk people who are asymptomatic.
To what extent is the device clinically translated?
It is not a diagnostic tool, but a research platform. Nevertheless, its ability to simulate pre-occlusive hemodynamics under controlled conditions enables its application to translational research (drug ranking, device concept testing). The project is being developed as an R&I action, which is now funded; partners now have the opportunity to develop the demonstrator.
How did the MIC (Microfluidics Innovation Center) contribute to the project?
MIC designed the microfluidic control layer, pressure control, bubble control, and assay coordination to ensure the biological model operates consistently. In practice, we have ensured that flow, shear, and cell viability are well within narrow limits, and that thrombotic phenomena can be quantified rather than confounded by the fluidics.
Does the platform have the ability to be adapted outside the carotids?
Yes. The same method can be used to simulate other large-artery beds or even microvascular niches by changing channel geometry and flow profiles. Replacing surface treatments or stents allows the teams to explore venous thrombosis, plaque rupture, erosion, and the interaction between devices and blood.
How does the inclusion of a specialist SME such as MIC in Horizon Europe consortia on vascular-on-chip issues?
Since the interpretation of biology depends upon the execution of engineering. MIC will unify microfabrication, controlled perfusion, automation, and documentation under one roof, having a record of EU collaborations. The inclusion of MIC as a microfluidics WP in our experience has doubled proposal success rates compared to official programme averages and shortened the time to deliver working prototypes that can be used to validate them.