Tips & Tricks for a successful HORIZON-HLTH-2026-01-DISEASE-11 proposal
Sex and/or gender-specific mechanisms of cardiovascular diseases
Opening
10 February 2026
Deadline
Keywords
Cluster health
risk factors
RIA
disease Prevention
cardiovascular diseases
gender-specific determinants
targeted interventions
hormone-linked mechanisms
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
HORIZON-HLTH-2026-01-DISEASE-11
This topic aims to generate actionable evidence on sex- and/or gender-specific determinants, risk factors, and pathways in cardiovascular diseases (CVDs), and to translate that knowledge into better prevention, detection, diagnosis, and treatment strategies.
Discover more!
Administrative facts of the HORIZON-HLTH-2026-01-DISEASE-11 call
Which call is it, and when is the opening and the deadline?
- Call name: Cluster 1 – Health (Single stage – 2026)
- Call identifier: HORIZON-HLTH-2026-01
- Destination: Tackling diseases and reducing disease burden
- Topic: HORIZON-HLTH-2026-01-DISEASE-11- Understanding of sex and/or gender-specific mechanisms of cardiovascular diseases: determinants, risk factors and pathways
- Opening date: 10 February 2026
- Deadline: 16 April 2026
- Type of Action: Research and Innovation Actions (RIA)
What about the budget and estimated size of the project?
- Overall topic budget: EUR 39.30 million
- Indicative number of funded projects: 6
- Budget/project: EUR 6.00-7.00 million
Scientific range: what the Commission expects from the HORIZON-HLTH-2026-01-DISEASE-11 grant?
Why is this topic important for the EU?
- CVDs remain a leading driver of mortality in Europe; Eurostat reported 1.71 million deaths in the EU in 2021 from circulatory diseases (32% of all deaths).
- The work programme stresses that sex and gender differences affect symptoms, prevalence, treatment response, and outcomes, and that women-specific conditions (e.g., menopause, pregnancy complications) can increase risk, so “one-size-fits-all” approaches miss prevention and care opportunities.
What outcomes are expected?
Proposals are expected to contribute to outcomes such as:
- Better understanding of sex- and/or gender-specific health determinants, risk factors, and pathways for CVDs (usable by researchers, developers of interventions, and healthcare professionals).
- Sex- and/or gender-tailored risk models that are actually used for improved prevention, detection, diagnosis, and treatment strategies.
- Healthcare systems are gaining novel sex- and/or gender-specific strategies that reduce CVD burden.
What scientific work is explicitly encouraged?
Your project should realistically cover most of the following directions (the closer you are, the safer you are):
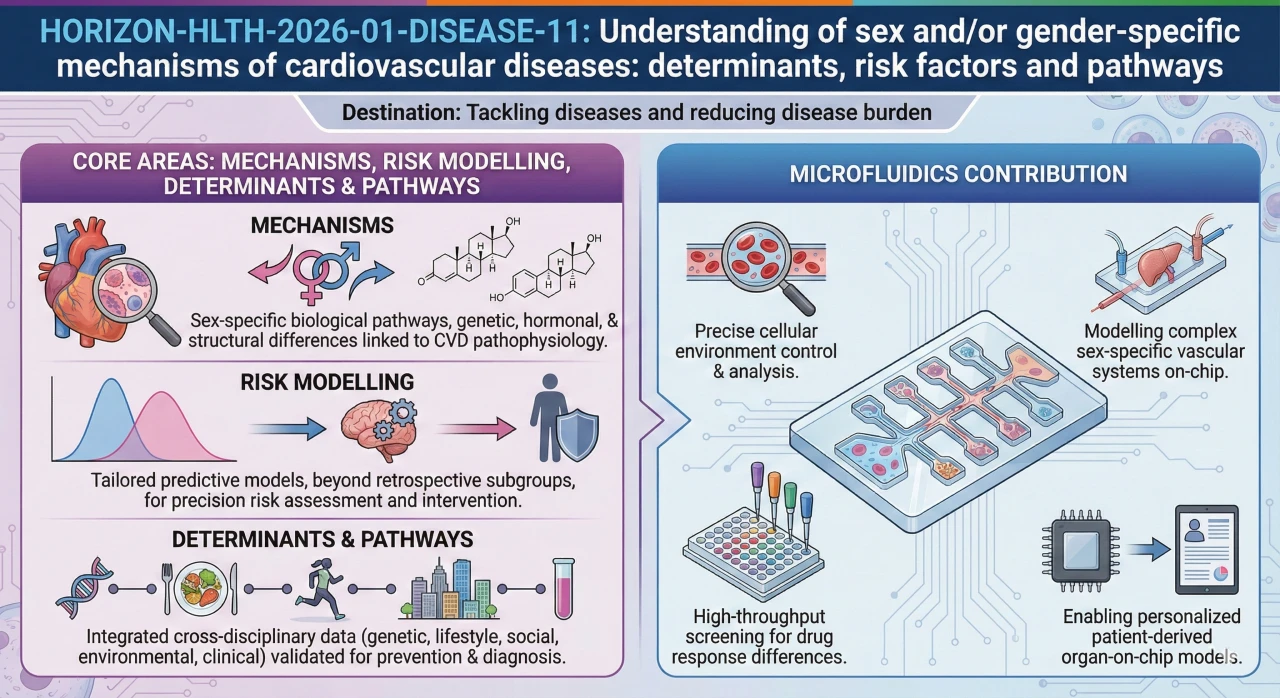
- Mechanisms: structural, hormonal, and/or biological distinctions between sexes/genders linked to CVD pathophysiology.
- Risk modelling: develop and validate sex- and/or gender-tailored risk models (not just retrospective subgroup plots).
- Determinants & pathways: identify and/or validate sex- and/or gender-specific determinants, risk factors, and pathways, using data that is:
- integrated across disciplines (molecular biology, behavioural science, nutrition, clinical research, social and environmental epidemiology, exposure sciences, genetics/epigenetics, etc.)
- validated (not only “signals”, but evidence strong enough to influence prevention/ diagnosis strategy).
Scientific strategy: How can you enhance your chances of being funded through HORIZON-HLTH-2026-01-DISEASE-11?
How do you “de-risk” the Excellence section?
- Don’t pitch “gender as a subgroup analysis.” Make it a mechanistic driver:
- Define sex variables (biological) and gender variables (sociocultural) and map each to hypotheses, endpoints, and datasets.
- Build a multi-layer causal chain:
- Determinants → pathways → biomarkers/phenotypes → risk models → prevention/diagnostic decisions.
- Make risk modelling credible:
- Plan external validation, calibration, fairness checks, and clinical interpretability (what changes in practice?).
- Treat “women-specific” and “life-stage” risks as first-class citizens:
- Pregnancy complications, menopause transition, hormone-related effects, and comorbidities.
What will evaluators likely see as a strong “Impact” story here?
- A credible bridge from mechanism → tool → adoption:
- Risk model outputs that can be integrated into guidelines, clinical decision pathways, or population screening logic.
- A pathway to reduce inequalities:
- Show how intersectional factors are incorporated to ensure risk models don’t underperform for underrepresented groups.
- A clear plan for what “healthcare system benefit” means:
- Measurable endpoints: improved detection, earlier prevention, better stratification, better outcomes.
What “hot buttons” are embedded in the topic text?
- FAIR data principles, registries/cohorts/biobanks
- Digital tech, including AI (not as buzzwords: specify where it adds value)
- SSH expertise for gender-related variables and behavioural/societal drivers
- Stakeholder relevance: clinicians + prevention actors + patient voices (to make strategies implementable)
Consortium & proposal-writing plan: what works best with this type of Health RIA?
Which consortium profile best fits this topic?
A strong DISEASE-11 consortium usually looks like a “triangle + bridge”:
- Triangle:
- Cardiovascular clinical expertise (cardiology departments, hospital networks)
- Epidemiology / public health (cohorts, registries, exposure science)
- Molecular / systems biology (omics, genetics/epigenetics, hormonal biology)
- Bridge:
- Data science / AI/biostatistics (risk modelling, validation, deployment)
- SSH experts (gender studies, behavioural science, health inequalities)
- Patient organisations/prevention stakeholders (uptake, relevance, ethics)
Discrete recommendation (because it helps): include an innovative SME that can turn risk models and evidence into deployable tools (clinical-grade software, decision support, analytics, validation pipelines). This also aligns with the work programme’s encouragement of SME participation.
What writing moves typically lift scores for this kind of RIA?
- Use “question-led” structure (mirrors evaluator thinking):
- What mechanism are we proving? → How will it change stratification? → What is the adoption path?
- Convert every major claim into:
- Method → dataset → metric → deliverable → user
- Make it painfully easy to evaluate:
- 1 page “logic model” (inputs → work packages → outputs → outcomes → impacts)
- a risk register that includes scientific risk + bias risk + data access risk
- Align work packages to the topic language:
- WP on determinants/pathways, WP on risk model development/validation, WP on translation to prevention/diagnosis strategy, WP on SSH & stakeholder uptake.
What common pitfalls should you avoid?
- “We will study sex differences” without:
- a mechanistic hypothesis, a validated model, and a route to clinical/public health action.
- Overpromising clinical impact without an implementation pathway.
- Treating SSH as dissemination only (it needs to shape design and interpretation).
How would microfluidics contribute to this topic?
Microfluidics can be a high-value “proof engine” for sex- and hormone-linked mechanisms, especially when human clinical data alone cannot isolate causality.
Practical ways microfluidics can help:
- Vessel-on-a-chip / endothelium-on-chip models to test:
- Sex-hormone modulation of endothelial dysfunction, inflammation, thrombosis, and permeability.
- Immune-vascular microphysiological systems to probe:
- Sex-differentiated immune signalling that affects plaque instability or microvascular disease.
- Cardiac tissue microfluidic platforms to study:
- Sex-dependent drug response/toxicity and electrophysiological differences.
- Microfluidic integration with omics:
- Generate controlled-condition multi-omics signatures to support biomarker discovery and mechanistic validation.
- Bridging to risk models:
- Use chip-derived mechanistic parameters as interpretable features or priors feeding predictive models (stronger biological plausibility, better generalisation).
Positioning tip:
- Present microfluidics not as “nice tech”, but as the causal validation layer that strengthens translation from determinants/pathways → sex/gender-tailored risk models → prevention/diagnosis strategy.
The MIC already brings its expertise in microfluidics to Horizon Europe:
H2020-NMBP-TR-IND-2020

Microfluidic platform to study the interaction of cancer cells with lymphatic tissue
H2020-LC-GD-2020-3

Toxicology assessment of pharmaceutical products on a placenta-on-chip model
FAQ - HORIZON-HLTH-2026-01-DISEASE-11
What is HORIZON-HLTH-2026-01-DISEASE-11?
It is a Research and Innovation Action (RIA) within the Health Cluster of Horizon Europe that will produce actionable evidence on sex- and/or gender-specific determinants, risk factors, and pathways in the cardiovascular diseases (CVDs) and translate the acquired knowledge into more effective prevention, detection, diagnosis, and treatment strategies.
What are the most important administrative particulars?
Opening date: 10 February 2026
Deadline: 16 April 2026
Total budget: EUR 39.30 million
Expected number of projects: 6
Budget per project: EUR 6 00-7 00 million.
Type of Action Research and Innovation Actions (RIA).
What is the significance of this issue to the EU?
In Europe, cardiovascular diseases are still a major killer of people, and in 2021, 1.71 million people died in the EU (32% of all deaths). Sex and gender disparities have important impacts on the symptoms of CVD, the prevalence, the response to treatment, and its outcomes. Certain conditions such as menopause or pregnancy complications are unique to women, thus women-specific interventions might be more effective but fail to take these vital prevention and care avenues into consideration.
What is expected in the way of scientific work?
Projects are supposed to cover a large portion of the following in realistically:
-Mechanisms: Determine the structural, hormonal, and biological differences between sexes/genders with CVD pathophysiology.
-Risk modeling: Test and establish sex or/and gender risk models.
-Determinants and pathways: Discover and confirm determinants that are sex- and/or gender-specific based on combined, multidisciplinary data (molecular biology, behavioral science, nutrition, clinical research, epidemiology, genetics/epigenetics).
What can the microfluidics do to this subject?
Microfluidics can be used as a proof engine of sex- and hormone-linked mechanisms:
-Vessel-on-a-chip/endothelium-on-chip models: Sex-hormone modulators of endothelial dysfunction, inflammation, and thrombosis.
-Immune-vascular microphysiological systems: Sex-differentiated immune signaling towards plaque instability.
-Microfluidic platforms of cardiac tissue: Experiment sex-specific drug response and electrophysiological variation.
-Microfluidic integration with omics: Create controlled multi-omics signatures in biomarker discovery.
-To risk models: Predictive models Use chip-derived mechanistic parameters as interpretable features.
What do you consider the pitfalls to avoid?
-The search to attempt to treat sex/gender as sub group analysis instead of as mechanistic drivers.
-Hyping clinical effects with no implementation routes.
-The experts in the field of SSH perceive the expertise as an aid in dissemination instead of part of study design.
-Absence of external validation, calibration and checks on fairness of the risk models.
-Failure to discuss the nature of the incorporation of risk models into clinical guidelines or practice.
What is the type of consortium that is likely to score well in DISEASE-11?
The suggestions that are comfortable to appraisers tend to have a triangular form with a bridge:
- Triangle (core science):
Cardiology clinical skills (hospital networks, clinical cohorts).
Epidemiology/public health (registries, population cohorts, exposure science).
Molecular/systems biology (omics, genetics/epigenetics, hormone biology).
- Bridge (translation/adoption):
Artificial intelligence/data science/biostatistics (risk model development, validation, design of deployment).
SSH knowledge (gender studies, behavioural science, inequalities) influencing study design- not only dissemination.
Patient organisations + prevention stakeholders (uptake, relevance, ethics, feasibility).
A discreet but often effective move: include an innovative SME that can turn evidence into something deployable (validated pipelines, decision-support prototypes, clinical-grade tooling, or lab-to-model integration). It tends to strengthen the “Impact and implementation” spine of the story. The Microfluidics Innovation Center is an SME specialized in microfluidic engineering and valorization of proposals in Horizon Europe.
