How to choose your recirculation system?
Author
Celeste Chidiac, PhD
Publication Date
Keywords
recirculation system
hydrostatic pressure
syringe pump
Peristaltic pump
pressure pump

Need advice for your recirculation system?
Your microfluidic SME partner for Horizon Europe
We take care of microfluidic engineering, work on valorization and optimize the proposal with you
Introduction
Fluid recirculation is essential for organ-on-a-chip and microfluidic applications, as it ensures the physiological transport of nutrients, drugs, and cell secretions.
Various systems are available, and choosing the most suitable option depends on the specific application, including factors like flow rate profile, accuracy, and user-friendliness. Recirculation systems can operate using hydrostatic pressure, a syringe pump, a peristaltic pump, or a pressure pump.
Characteristics/ Recirculation systems | Flow directionality | Flow rate control | Flow profile |
Hydrostatic pressure | Normally bidirectional with a rocker | None to limited; Can be controlled using a programmable rocking platform | Affected by rocker speed and tilt angle, and/ or reservoir height and liquid volume |
Infuse/withdraw syringe pump | Normally bidirectional | Controlled by the pump | Amplitude of screw-motion; pulsatility depends on pump |
Peristaltic pump | Unidirectional flow | Controlled by the pump | Moderately pulsatile flow; Can be reduced with a dampener |
Pressure pump and valve | Unidirectional flow with the use of a valve | Precise flow rate control; Uses a flow sensor feedback loop | Highly stable profile; Variety of flows possible: pulsatile, steady, stepwise, custom |
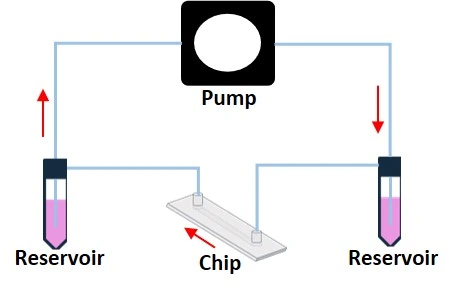
Hydrostatic pressure recirculation system
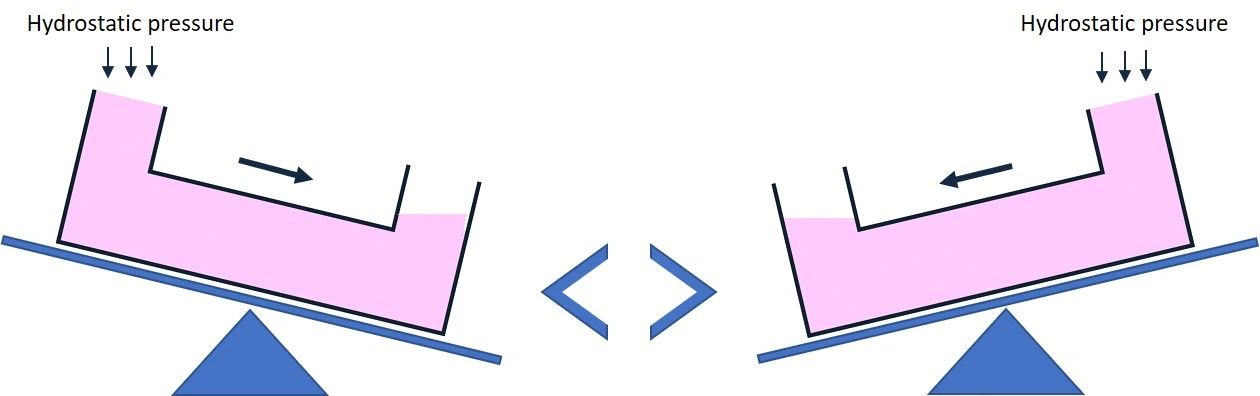
- Mechanism: Flow is generated by a height difference between the inlet and outlet. Switching the height difference reverses the flow direction.
- Characteristics/Considerations:
- Simple to implement, requiring no electronics.
- Cost-effective and accessible, suitable for basic setups.
- Bidirectional flow.
- A rocking platform can be used to control the back-and-forth fluid movement.
- Difficulty in precisely controlling or predicting flow rates.
- Flow rate estimation is highly variable, limiting reproducibility.
- Applications: Ideal for preliminary experiments or where precision is less critical.
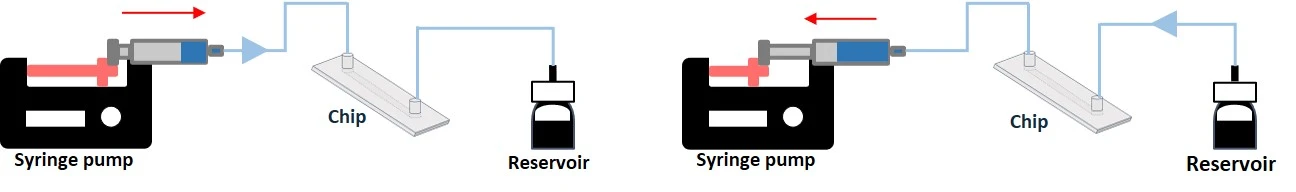
Syringe pump recirculation system
- Mechanism: Syringe pumps push or pull fluid through a microfluidic device with programmable flow rates. Bidirectional systems use multi-syringe setups for continuous push-pull cycles.
- Characteristics/Considerations:
- Programmable flow rate control.
- Bidirectional flow with a push/pull system.
- Flow oscillations caused by motor steps can affect sensitive applications.
- Syringe volume limits the duration of experiments; frequent refilling may disrupt workflows.
- Applications: Well-suited for long-term experiments needing precise flow control, such as cell culture studies.
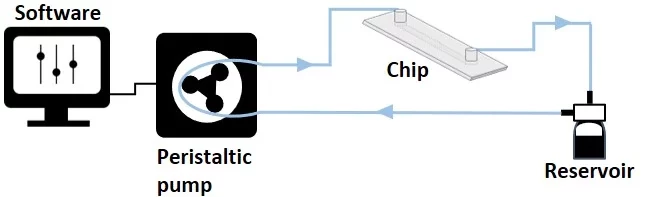
Peristaltic pump recirculation system
- Mechanism: Fluid circulates through a closed-loop system, maintaining steady liquid levels.
- Characteristics/Considerations:
- Simple and user-friendly setup for unidirectional flows.
- Generates pulsatile flow due to the pump’s rotor motion, which may affect particle or cell integrity.
- Pulsation dampeners can be added to achieve smoother flows.
- Applications: Used in cell culture and particle studies where unidirectional flow is needed or pulsatile flow is acceptable.
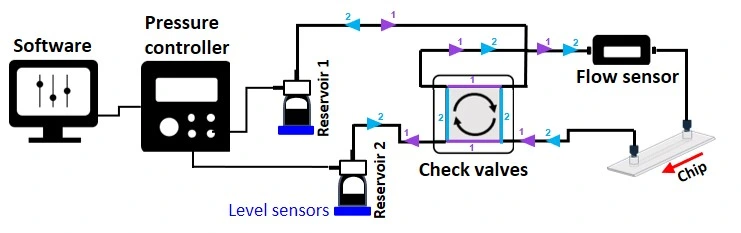
Pressure-driven recirculation system
- Mechanism: Flow is created by a pressure differential (ΔP) across the circuit, controlled by a pressure pump.
- Characteristics/Considerations:
- Configurations include bidirectional open-loop systems or unidirectional setups with valve integration.
- High precision and stability in flow rates, enabled by feedback-controlled pressure systems.
- Versatility in controlling flow paths using valve systems.
- Requires technical expertise and equipment, including flow sensors and controllers.
- More costly and complex compared to simpler systems.
- Applications: Ideal for advanced and highly controlled experiments, such as organ-on-a-chip systems with strict physiological requirements.
Want to know more on this subject? Check the extended review comparing the different recirculation systems.
Conclusion
The choice of a suitable recirculation system should be guided by the application’s specific requirements. Hydrostatic pressure systems offer a simple and cost-effective solution for basic experiments, while syringe pumps provide precise flow control for long-term studies, with limitations in duration and flow smoothness. Peristaltic pumps are user-friendly and suitable for applications tolerating pulsatile flow, and pressure-driven systems excel in precision and flexibility for advanced setups. By aligning the characteristics of these systems with experimental needs, researchers can optimize the performance and reproducibility of their microfluidic and organ-on-a-chip studies.
Funding and Support
This review was written under funding from the European Union’s H2020-LC-GD-2020-3, grant agreement no. 101037090 (ALTERNATIVE, project page).


This “quick tips” was written by Celeste Chidiac, PhD.
Published in January 2025.
Contact: Partnership[at]microfluidic.fr

Check more Quick Tips
FAQ - How to choose your recirculation system?
What do they actually mean by a recirculation system in microfluidics?
In practice, a recirculation system allows the same volume of fluid to circulate repeatedly through a microfluidic device, rather than constantly using fresh medium. This approach is especially important for organ-on-a-chip platforms and long-term cultures, where maintaining a stable microenvironment is critical. Recirculation enables nutrients to be replenished, metabolites and secreted factors to accumulate naturally, and drugs to be administered at physiologically relevant concentrations.
The chosen pumping strategy directly affects the system’s performance, influencing flow stability, shear stress, bubble formation, sterility, and reproducibility over time. In short, effective recirculation is key to making microfluidic experiments both realistic and reliable.
In simplistic terms, what are the key recirculation strategies?
The majority of laboratory configurations are in four families:
-Hydrostatic pressure: may involve reservoirs of varying heights and a rocker.
-Syringe pump: Infuse/withdraw (push-pull, usually bidirectional).
-Peristaltic pump: closed loop, normally unidirectional.
-Pressure-driven flow: pressure controller + sensors, frequently with valves in routing.
In case I remember only one thing, what will direct the selection?
Begin with your biological or physical necessity, not with the pump catalogue. It is normally sorted out by three questions:
Is bidirectional flow required, or is unidirectional flow sufficient?
What flow was required to be steady (vs intentionally pulsatile vs stepwise/custom), or not?
What level of control are you actually required to have: “approximately speaking the correct order of magnitude” or “I will have to justify this number in a paper”?
What is a hydrostatic (gravity /rocking) system? When is it the tool of the trade?
Hydrostatic recirculation is good when you need something straightforward, quick, and cheap, no electronics, few modes of malfunction, and it is easy to teach a new student. When you do it on a rocking platform, you usually achieve a back-and-forth flow that is suitable for early prototyping or screening.
But simplicity is expensive: the flow rate is difficult to model and may be biased by reservoir height, liquid volume, angle of inclination, and even leveling errors.
Is it bad idea to use peristaltic pumps on cells due to pulsation?
Not automatically. The reason peristaltic pumps are popular is that they are simple, easy to use, and inherently suited to unidirectional closed loops with steady liquid levels. Trade-off: Pulsatile flow due to the roller action can be a source of stress for the cells or disrupt the paths of the particles in certain assays.
The sensible trade-off would be to incorporate a pulsation-damping device (or a compliant portion of the tubing). That will not make it an ideal pressure controller, but it frequently causes the flow to be biologically acceptable to most culture processes.
At what level of complexity is it worthwhile to use pressure-driven systems?
Recirculation with a pressure-driven system is what you choose when flow is an experimental variable you are interested in. The pressure controller has a flow sensor for feedback, provides very stable flow, and lets you create intentional profiles (steady, pulsatile, stepwise, custom) and intelligent routing (valves can be used for unidirectional recirculation).
The method is more expensive, involves more components, and requires more troubleshooting. However, it pays off in terms of reproducibility and physiological control, particularly in the more advanced studies of organs-on-a-chip.
What is the decision-making between bidirectional and unidirectional recirculation?
Bidirectional flow, such as that generated by rocking platforms or push/pull syringes, can be sufficient for some barrier models or screening assays. However, it disrupts gradients and alternates the direction of shear stress, which can interfere with sensitive biological readouts.
Unidirectional flow, achieved with peristaltic pumps or pressure-driven systems with valves, more closely mimics physiological tissue perfusion. It preserves stable gradients, consistent dosing profiles, and downstream analytics, making it the safer choice when maintaining oxygen, drug, or cytokine gradients is critical.
In short, unidirectional flow usually reduces experimental variability and prevents unwanted artifacts in recirculating microfluidic systems.
What are the criteria people forget until the experiment fails?
-Bubble control (degassing plan, bubble traps, gas-permeable tube options)
-Stability between sterilizations (connectors, sampling ports, and the frequency of human contact with the loop).
-Adsorption /absorption (in particular with small hydrophobic drugs in select tubing materials)
-Position of the sensor (flow sensors may be pulsation sensitive, bubble sensitive, temperature sensitive)
-Dead volume, residence time (which may surreptitiously alter dosing dynamics)
We are developing a Horizon Europe proposal based on organ-on-a-chip. What does the work plan need in regard to recirculation choices?
There are two things that are liked by reviewers (1) you know what your control variables are, and (2) you have a plausible path to partner reproducibility. A neat way to frame it is:
-Early stage: a powerful, basic recirculation system of rapid prototyping and minimization of risks.
-Validation stage: a more rigorous setup for physiological relevance/inter-lab reproducibility.
-Consortium design: adding an established microfluidic SME can eliminate much technical vagueness in the proposal (chip design, setup engineering, automation, prototyping, and how this will be built and tested).
-Backing up competitiveness: On average, the success rate of proposals submitted to Horizon Europe has been reported at around 16%. In such a climate, everything that makes execution look more realistic, particularly from an industrial R&D partner capable of prototyping, will be important.
What is the typical contribution of MIC when a consortium needs properly implemented recirculation?
The key challenge is designing the entire fluidic chain so that the biology team can focus on their experiments, rather than troubleshooting fluidics. MIC supports this by defining the recirculation architecture, selecting chips and connectors, integrating valves and sensors where needed, designing automation, and delivering a system that is reproducible and validated across multiple sites.
Equally important for EU projects is translating this work into a coherent, credible work package: clear deliverables, identified risks with mitigation strategies, and realistic prototype milestones. By participating as an R&D partner in European consortia, MIC helps maintain a seamless technical storyline from proposal through prototyping, ensuring the project remains both technically sound and evaluable.

